
$C{H_3}Cl$ has more dipole moment than $C{H_3}F$ because:
(A) Electron affinity of chlorine is greater than that of fluorine
(B) The charge separation is larger in $C{H_3}Cl$ compared to $C{H_3}F$
(C) The repulsion between the bond pairs and non – bonded pairs of electrons is greater in $C{H_3}Cl$ than $C{H_3}F$
(D) Chlorine has higher electronegativity than fluorine
Answer
582.3k+ views
Hint:Dipole moment is the result of charge separation between positive and negative charges. Dipole moment is calculated by the product of charge and distance of separation.
$\mu = q \times d$
$\mu $= dipole moment
q = charge
d = distance of separation
Its units are coulomb meter or debye.
Complete step by step answer:The structure of $C{H_3}Cl$ is:
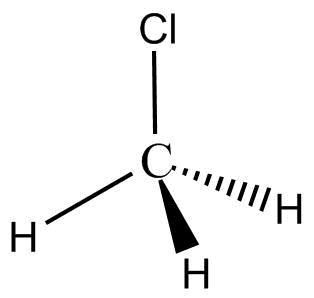
Its IUPAC name is methyl chloride. Its shape is tetrahedral. In $C{H_3}Cl$ carbon is the central atom three hydrogens and one chlorine is arranged around carbon. $C{H_3}Cl$ Molecule is a dipole molecule as carbon is more electronegative than hydrogen. Therefore, the shared pair of electrons move towards carbon and hydrogen obtain a partial positive charge.
In turn, chlorine is more electronegative than carbon thus it attracts the shared electrons and gets partial negative charge. Thus, the molecule has both partial positive and partial negative charge. The bond angle in $C{H_3}Cl$ is ${109.5^\circ }$
According to the formula of dipole moment, it depends on both charge and distance between charges. Fluorine is more electronegative than chlorine, but $C - Cl$ bond is larger than $C - F$ bond is $178$ and $139pm$ respectively.
Therefore, by considering both the points due to large distance of separation in $C{H_3}Cl$ as compared to $C{H_3}F$, the dipole moment of $C{H_3}Cl$ is greater than $C{H_3}F$.
Hence, the correct option is (B) the charge separation is larger in $C{H_3}Cl$ compared to $C{H_3}F$.
Note:The dipole moment of a single bond in a polyatomic molecule is very different from the dipole moment in a molecule as a whole. It is a vector quantity and also can be zero.
$\mu = q \times d$
$\mu $= dipole moment
q = charge
d = distance of separation
Its units are coulomb meter or debye.
Complete step by step answer:The structure of $C{H_3}Cl$ is:

Its IUPAC name is methyl chloride. Its shape is tetrahedral. In $C{H_3}Cl$ carbon is the central atom three hydrogens and one chlorine is arranged around carbon. $C{H_3}Cl$ Molecule is a dipole molecule as carbon is more electronegative than hydrogen. Therefore, the shared pair of electrons move towards carbon and hydrogen obtain a partial positive charge.
In turn, chlorine is more electronegative than carbon thus it attracts the shared electrons and gets partial negative charge. Thus, the molecule has both partial positive and partial negative charge. The bond angle in $C{H_3}Cl$ is ${109.5^\circ }$
According to the formula of dipole moment, it depends on both charge and distance between charges. Fluorine is more electronegative than chlorine, but $C - Cl$ bond is larger than $C - F$ bond is $178$ and $139pm$ respectively.
Therefore, by considering both the points due to large distance of separation in $C{H_3}Cl$ as compared to $C{H_3}F$, the dipole moment of $C{H_3}Cl$ is greater than $C{H_3}F$.
Hence, the correct option is (B) the charge separation is larger in $C{H_3}Cl$ compared to $C{H_3}F$.
Note:The dipole moment of a single bond in a polyatomic molecule is very different from the dipole moment in a molecule as a whole. It is a vector quantity and also can be zero.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




