
What is the charge on the polyatomic ions nitrite and chlorite?
Answer
521.1k+ views
Hint :We know that for solving this problem, you should have the knowledge about polyatomic ion and oxidation number.
A Polyatomic ion is an ion containing more than one atom. Oxidation number is the number of electrons lost or gained by an element during a reaction. Charges with respect to the Lewis structure, the charge on a polyatomic ion can be identified easily.
Complete Step By Step Answer:
Ions are those molecules or compounds that have either a positive or negative charge. They are classified as monatomic in which only one atom is present with either positive or negative charge, diatomic in which two atoms are present, and polyatomic in which more than two atoms are present. Lewis structure helps in representing valence electrons of a molecule. On calculating the formal.
Step-1: To calculate the charge on a polyatomic ion, the oxidation number of all the atoms can be added together.
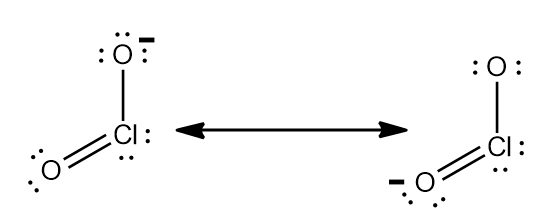
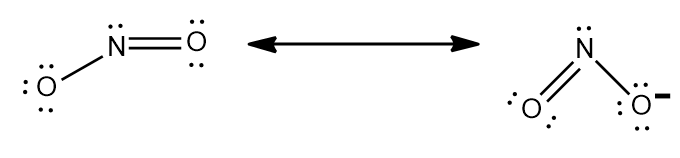
Step-2: Nitrite ion stands for $ NO_{2}^{-} $ .So, charge can be calculated as sum of oxidation number of nitrogen and oxygen.
Similarly Chlorite ion stands for $ ClO_{2}^{-} $ .
Step-3: The charge can also be calculated using Lewis structure and making sure every atom in the ion has $ 8 $
electrons. Using the given phenomenon and studying the Lewis structure of $ NO_{2}^{-} $ and $ ClO_{2}^{-} $ ,they both
$ -1 $ charge.
Note :
There are many ways to identify the charge on a polyatomic ion and one can use any way they find easy to remember. But making the correct Lewis structure is an important step which must not be forgotten. The overall ionic formula of the compound must be electrically neutral, that is it should have zero charges and while writing the formula of the compound we assure that the cation part should come first followed by anion.
A Polyatomic ion is an ion containing more than one atom. Oxidation number is the number of electrons lost or gained by an element during a reaction. Charges with respect to the Lewis structure, the charge on a polyatomic ion can be identified easily.
Complete Step By Step Answer:
Ions are those molecules or compounds that have either a positive or negative charge. They are classified as monatomic in which only one atom is present with either positive or negative charge, diatomic in which two atoms are present, and polyatomic in which more than two atoms are present. Lewis structure helps in representing valence electrons of a molecule. On calculating the formal.
Step-1: To calculate the charge on a polyatomic ion, the oxidation number of all the atoms can be added together.


Step-2: Nitrite ion stands for $ NO_{2}^{-} $ .So, charge can be calculated as sum of oxidation number of nitrogen and oxygen.
Similarly Chlorite ion stands for $ ClO_{2}^{-} $ .
Step-3: The charge can also be calculated using Lewis structure and making sure every atom in the ion has $ 8 $
electrons. Using the given phenomenon and studying the Lewis structure of $ NO_{2}^{-} $ and $ ClO_{2}^{-} $ ,they both
$ -1 $ charge.
Note :
There are many ways to identify the charge on a polyatomic ion and one can use any way they find easy to remember. But making the correct Lewis structure is an important step which must not be forgotten. The overall ionic formula of the compound must be electrically neutral, that is it should have zero charges and while writing the formula of the compound we assure that the cation part should come first followed by anion.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




