
How many chelate rings are present in each of the following complexes?
${{[Cu(trien)]}^{2+}}$ , ${{[Fe{{(ox)}_{3}}]}^{3-}}$ , ${{[Ru{{(bpy)}_{3}}]}^{2+}}$ , and ${{[Co{{(dien)}_{2}}]}^{3+}}$ .
Answer
535.5k+ views
Hint: Ligands are a group of atoms attached with the central atom in a complex. Chelate ligands are formed when these atoms bind to the central atom.
Complete answer:
A ligand is classified as an unidentate that has only one donor site, while bi or poly dentate ligands have more than one donor site. When these bi or poly dentate ligands attach or bind together with the central atom, they form chelate rings in a complex. The number of chelate ligands or groups is also called the denticity of a ligand.
We have been given to find the number of chelate rings of the given complexes.
${{[Cu(trien)]}^{2+}}$, this complex contains trien (triethylenetetramine) as a ligand. The ligand has denticity of 3, so the binding will occur and the structure becomes,
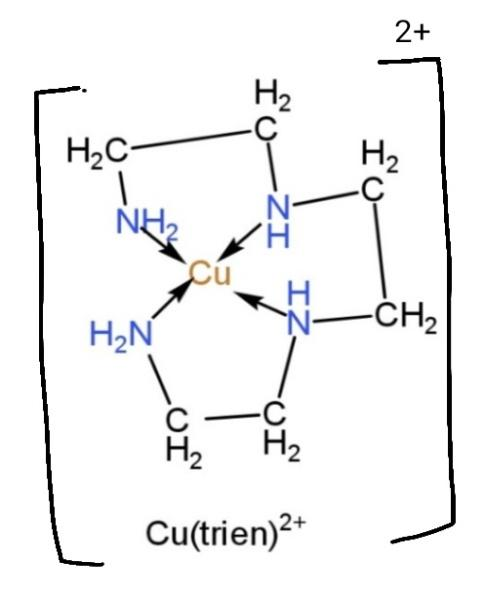
Clearly, the structure contains 3 chelate rings.
${{[Fe{{(ox)}_{3}}]}^{3-}}$, contain oxalate as a ligand, with denticity 3, and it bind though two atoms, so the structure become,
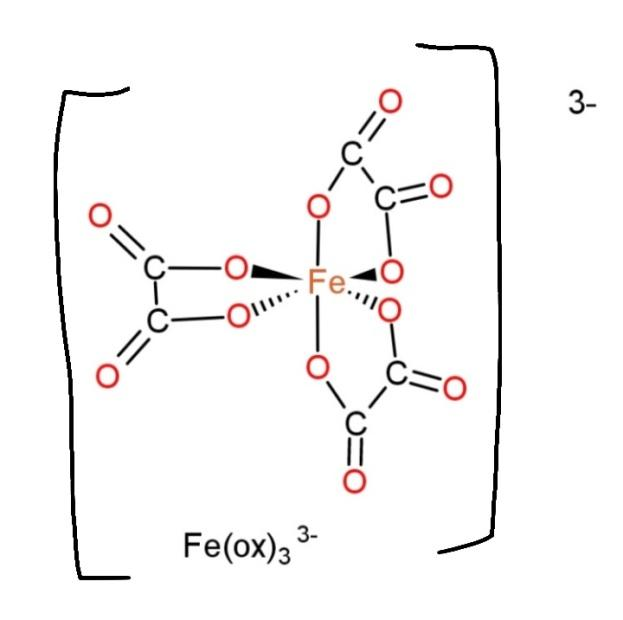
This structure shows us 3 distinct chelate rings.
${{[Ru{{(bpy)}_{3}}]}^{2+}}$, contains bipyridine as ligand with denticity of 3, this can bind through both of their nitrogens to for the structure,
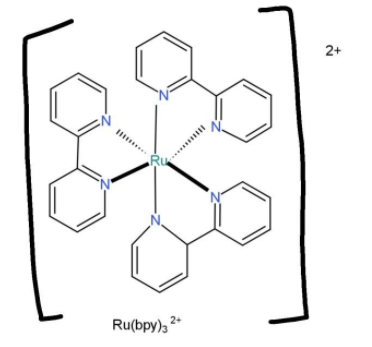
As the ligand when attached with the central atom forms 3 rings, so chelate rings are 3.
${{[Co{{(dien)}_{2}}]}^{3+}}$, contains diethylenetriamine as ligand, with denticity of 2, and this binds with the central atom as,
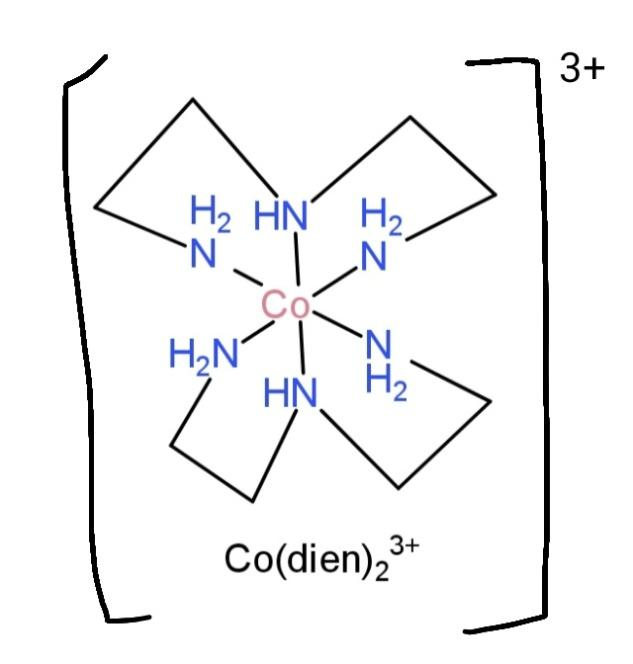
So, this structure makes up 4 distinct chelate rings.
Hence, the number of chelate rings are 3, 3, 3, and 4 in ${{[Cu(trien)]}^{2+}}$ , ${{[Fe{{(ox)}_{3}}]}^{3-}}$ , ${{[Ru{{(bpy)}_{3}}]}^{2+}}$ , and ${{[Co{{(dien)}_{2}}]}^{3+}}$respectively.
Note:
In order to determine the exact number of chelate rings, the complex needs to be made properly. This can be achieved by knowing the structures of ligands and their attachment along with their denticity. The chelate rings may be seen as distinctly when the ligands bind with the central metal.
Complete answer:
A ligand is classified as an unidentate that has only one donor site, while bi or poly dentate ligands have more than one donor site. When these bi or poly dentate ligands attach or bind together with the central atom, they form chelate rings in a complex. The number of chelate ligands or groups is also called the denticity of a ligand.
We have been given to find the number of chelate rings of the given complexes.
${{[Cu(trien)]}^{2+}}$, this complex contains trien (triethylenetetramine) as a ligand. The ligand has denticity of 3, so the binding will occur and the structure becomes,

Clearly, the structure contains 3 chelate rings.
${{[Fe{{(ox)}_{3}}]}^{3-}}$, contain oxalate as a ligand, with denticity 3, and it bind though two atoms, so the structure become,

This structure shows us 3 distinct chelate rings.
${{[Ru{{(bpy)}_{3}}]}^{2+}}$, contains bipyridine as ligand with denticity of 3, this can bind through both of their nitrogens to for the structure,

As the ligand when attached with the central atom forms 3 rings, so chelate rings are 3.
${{[Co{{(dien)}_{2}}]}^{3+}}$, contains diethylenetriamine as ligand, with denticity of 2, and this binds with the central atom as,

So, this structure makes up 4 distinct chelate rings.
Hence, the number of chelate rings are 3, 3, 3, and 4 in ${{[Cu(trien)]}^{2+}}$ , ${{[Fe{{(ox)}_{3}}]}^{3-}}$ , ${{[Ru{{(bpy)}_{3}}]}^{2+}}$ , and ${{[Co{{(dien)}_{2}}]}^{3+}}$respectively.
Note:
In order to determine the exact number of chelate rings, the complex needs to be made properly. This can be achieved by knowing the structures of ligands and their attachment along with their denticity. The chelate rings may be seen as distinctly when the ligands bind with the central metal.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

10 examples of friction in our daily life




