
What is the chemical formula for the polyatomic ion acetate?
Answer
527.7k+ views
Hint: Chemical formula is going to explain the proportions of the chemicals which are present in a particular compound. The chemical symbols of the elements which are present in a particular compound going to be represented in the chemical formula.
Complete answer:
- In the question it is asked to write the chemical formula for the polyatomic ion acetate.
- First we should know about the acetate ion before we are going to know about the polyatomic ion acetate.
- The chemical formula to represent the acetate ion is as follows.
\[C{{H}_{3}}COOH\rightleftharpoons \underset{Acetate\text{ }ion}{\mathop{C{{H}_{3}}CO{{O}^{-}}}}\,+{{H}^{+}}\]
- Acetate anion is going to be generated from the acetic acid.
- Acetate anion also called as a conjugate base of acetic acid.
- The salt of the acetic acid is called acetate ion.
- Two moles of the acetic acid undergo condensation reaction and form a chemical called polyatomic ion acetate.
- The chemical reaction of converting acetic acid to polyatomic ion acetate is as follows.
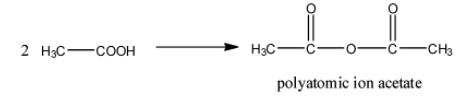
- From the above chemical reaction we can write the chemical formula of the polyatomic ion acetate.
- The chemical formula of polyatomic ion acetate is $(C{{H}_{3}}CO)O$ .
Note:
Acetate ions undergo condensation reaction and form polyatomic ion acetate and it is also called as acetic anhydride. Acetic anhydride has a big role in chemical reactions to do acetylation reactions to prepare acetylated products. In the synthesis of aspirin acetic anhydride has a crucial role.
Complete answer:
- In the question it is asked to write the chemical formula for the polyatomic ion acetate.
- First we should know about the acetate ion before we are going to know about the polyatomic ion acetate.
- The chemical formula to represent the acetate ion is as follows.
\[C{{H}_{3}}COOH\rightleftharpoons \underset{Acetate\text{ }ion}{\mathop{C{{H}_{3}}CO{{O}^{-}}}}\,+{{H}^{+}}\]
- Acetate anion is going to be generated from the acetic acid.
- Acetate anion also called as a conjugate base of acetic acid.
- The salt of the acetic acid is called acetate ion.
- Two moles of the acetic acid undergo condensation reaction and form a chemical called polyatomic ion acetate.
- The chemical reaction of converting acetic acid to polyatomic ion acetate is as follows.

- From the above chemical reaction we can write the chemical formula of the polyatomic ion acetate.
- The chemical formula of polyatomic ion acetate is $(C{{H}_{3}}CO)O$ .
Note:
Acetate ions undergo condensation reaction and form polyatomic ion acetate and it is also called as acetic anhydride. Acetic anhydride has a big role in chemical reactions to do acetylation reactions to prepare acetylated products. In the synthesis of aspirin acetic anhydride has a crucial role.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




