
What is the chemical formula of calcium $ (II) $ nitrate?
Answer
522.3k+ views
Hint :Knowing the name of the elements and few basic rules allows us to name simple compounds given the chemical formula. We also can reverse the process. That is, if we know the name of the compound we should be able to write the chemical formula.
Complete Step By Step Answer:
Calcium nitrate is also known as Norges Saltpeter. It was initially synthesized at Notodden, which is in Norway in $ 1905 $ by the Birkeland-Eyde process. In Porsgrunn, most of the calcium nitrate is made. When limestone is treated with nitric acid, followed by neutralization with ammonia calcium nitrate is formed. In the environment, Calcium nitrate can also control certain plant diseases. For example, dilute calcium nitrate sprays are used to control bitter pit and cork spots in apple trees.
Additionally it helps in consuming easy degradable organic matter, which otherwise can lead to anaerobic conditions downstream as well odour emission itself. A chemical formula shows that the element that forms compound and the attached atoms to the elements are small units of that compound, be it a molecule or a formula unit. To write down the calcium nitrate formula, we will first determine the valences of the two elements present that is calcium and nitrate. Calcium has $ + 2 $ and Nitrate has $ - 1 $ respectively. Now put these numbers below the symbols. Follow the below diagram
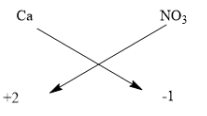
This way the formula is
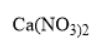
Note :
Calcium nitrate is used in wastewater preconditioning for odour emission presentation. The wastewater pre-conditioning is based on establishing an anoxic biology in the wastewater system. In the presence of nitrate, it helps in preventing formation of hydrogen sulphide because in its presence the metabolism for sulphur stops.
Complete Step By Step Answer:
Calcium nitrate is also known as Norges Saltpeter. It was initially synthesized at Notodden, which is in Norway in $ 1905 $ by the Birkeland-Eyde process. In Porsgrunn, most of the calcium nitrate is made. When limestone is treated with nitric acid, followed by neutralization with ammonia calcium nitrate is formed. In the environment, Calcium nitrate can also control certain plant diseases. For example, dilute calcium nitrate sprays are used to control bitter pit and cork spots in apple trees.
Additionally it helps in consuming easy degradable organic matter, which otherwise can lead to anaerobic conditions downstream as well odour emission itself. A chemical formula shows that the element that forms compound and the attached atoms to the elements are small units of that compound, be it a molecule or a formula unit. To write down the calcium nitrate formula, we will first determine the valences of the two elements present that is calcium and nitrate. Calcium has $ + 2 $ and Nitrate has $ - 1 $ respectively. Now put these numbers below the symbols. Follow the below diagram

This way the formula is
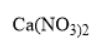
Note :
Calcium nitrate is used in wastewater preconditioning for odour emission presentation. The wastewater pre-conditioning is based on establishing an anoxic biology in the wastewater system. In the presence of nitrate, it helps in preventing formation of hydrogen sulphide because in its presence the metabolism for sulphur stops.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




