
Chemical formula of red lead is:
A. PbO
B. $\text{Pb}{{\text{O}}_{\text{2}}}$
C. $\text{P}{{\text{b}}_{3}}{{\text{O}}_{4}}$
D. $\text{P}{{\text{b}}_{2}}{{\text{O}}_{3}}$
Answer
586.5k+ views
Hint: We need to know the structure of red lead at first. Then we can count the number of oxygen and lead atoms in the compound.
Complete step by step answer:
Red lead is also known as minium. It is an organic compound and it has a bright reddish orange colour. Red lead is very useful in the manufacture of batteries, rust proof primer paints, etc. It is also an example of a mixed valence compound since it is composed of both lead (II) and lead (IV) in the ratio of 2:1. Lead (II)(IV) oxide has a tetragonal crystal structure at room temperature and it transforms to an orthorhombic at temperatures of 170 K or $-103{}^\circ C$. This translation of its phase, changes only the symmetry of the crystal and slightly modifies the interatomic distances and angles. This compound is prepared by the calcination of lead (II) oxide in air at about $\text{450 }\!\!{}^\circ\!\!\text{ C}$ to $\text{480 }\!\!{}^\circ\!\!\text{ C}$. The chemical reaction for the same is given below:
\[\text{6 }\!\!~\!\!\text{ PbO + }{{\text{O}}_{\text{2}}}\text{ }\!\!~\!\!\text{ }\xrightarrow{{}}\text{ 2P}{{\text{b}}_{\text{3}}}{{\text{O}}_{\text{4}}}\]
The chemical structure of red lead is given below from which we can count that there are 3 atoms of lead centred with 4 neighbouring atoms of oxygen hence forming the equation for red lead as $\text{P}{{\text{b}}_{3}}{{\text{O}}_{4}}$. Having a melting point of around $\text{500 }\!\!{}^\circ\!\!\text{ C}$, this compound exists in the form of vivid orange crystals.
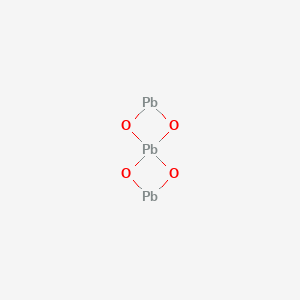
Therefore, the chemical formula for red lead is $\text{P}{{\text{b}}_{3}}{{\text{O}}_{4}}$
So, the correct answer is “Option C”.
Note: Natural red lead is very rare and it is formed only in extreme oxidizing conditions of lead ore bodies. The red lead is virtually insoluble in water and also in ethanol, though it is soluble in hydrochloric acid present in the stomach and therefore very toxic when ingested. It also dissolves in glacial acetic acid and diluted mixture of nitric acid and hydrogen peroxide also. When it is heated to a temperature of about $500{}^\circ C$ it decomposes to lead (II) oxide and oxygen, and at the temperature of $580{}^\circ C$, the reaction gets complete. The chemical equation for the same is given below:
\[\text{2P}{{\text{b}}_{\text{3}}}{{\text{O}}_{\text{4}}}\text{ }\!\!~\!\!\text{ }\xrightarrow{{}}\text{ 6PbO + }{{\text{O}}_{\text{2}}}\]
Complete step by step answer:
Red lead is also known as minium. It is an organic compound and it has a bright reddish orange colour. Red lead is very useful in the manufacture of batteries, rust proof primer paints, etc. It is also an example of a mixed valence compound since it is composed of both lead (II) and lead (IV) in the ratio of 2:1. Lead (II)(IV) oxide has a tetragonal crystal structure at room temperature and it transforms to an orthorhombic at temperatures of 170 K or $-103{}^\circ C$. This translation of its phase, changes only the symmetry of the crystal and slightly modifies the interatomic distances and angles. This compound is prepared by the calcination of lead (II) oxide in air at about $\text{450 }\!\!{}^\circ\!\!\text{ C}$ to $\text{480 }\!\!{}^\circ\!\!\text{ C}$. The chemical reaction for the same is given below:
\[\text{6 }\!\!~\!\!\text{ PbO + }{{\text{O}}_{\text{2}}}\text{ }\!\!~\!\!\text{ }\xrightarrow{{}}\text{ 2P}{{\text{b}}_{\text{3}}}{{\text{O}}_{\text{4}}}\]
The chemical structure of red lead is given below from which we can count that there are 3 atoms of lead centred with 4 neighbouring atoms of oxygen hence forming the equation for red lead as $\text{P}{{\text{b}}_{3}}{{\text{O}}_{4}}$. Having a melting point of around $\text{500 }\!\!{}^\circ\!\!\text{ C}$, this compound exists in the form of vivid orange crystals.

Therefore, the chemical formula for red lead is $\text{P}{{\text{b}}_{3}}{{\text{O}}_{4}}$
So, the correct answer is “Option C”.
Note: Natural red lead is very rare and it is formed only in extreme oxidizing conditions of lead ore bodies. The red lead is virtually insoluble in water and also in ethanol, though it is soluble in hydrochloric acid present in the stomach and therefore very toxic when ingested. It also dissolves in glacial acetic acid and diluted mixture of nitric acid and hydrogen peroxide also. When it is heated to a temperature of about $500{}^\circ C$ it decomposes to lead (II) oxide and oxygen, and at the temperature of $580{}^\circ C$, the reaction gets complete. The chemical equation for the same is given below:
\[\text{2P}{{\text{b}}_{\text{3}}}{{\text{O}}_{\text{4}}}\text{ }\!\!~\!\!\text{ }\xrightarrow{{}}\text{ 6PbO + }{{\text{O}}_{\text{2}}}\]
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

Explain zero factorial class 11 maths CBSE

What organs are located on the left side of your body class 11 biology CBSE

Draw a diagram of nephron and explain its structur class 11 biology CBSE




