
How many chiral carbons are present in glucose molecules $CHO{{(CHOH)}_{4}}C{{H}_{2}}OH$?
(A) 4
(B) 3
(C) 2
(D) 1
Answer
584.1k+ views
Hint: Revise the concept of chirality. Find out the meaning and criteria of assigning a chiral carbon or an asymmetric carbon atom. Draw the structure of glucose in Fisher projection formula and then mark the chiral carbons present in glucose. Count all the chiral carbons to get the answer.
Complete step by step solution:
- Chirality is the property of asymmetry in a molecule or any substance.
- If the mirror image of a substance is non-superimposable with that substance then, that substance is said to possess chirality and the substance is said to be chiral or asymmetric. It is represented by an asterisk (*).
- For example the letter ‘A’ has the mirror image ‘A’ only so both the images can be superimposed as one image. So, the letter ‘A’ is not chiral or achiral.
- For letter ‘B’ or ‘D’ or ‘P’, its mirror images are non-superimposable to each other and therefore, these letters are chiral and enantiomers of each other.
- Another example is of hand, look at the palms of both of your hands. They are mirror images of each other. But when you keep your right hand palm on your left hand palm, like stacking, they do not superimpose on each other. You can see that at least both your thumbs are on different sides. Therefore, palms are chiral and they are enantiomers of each other.
- In chemistry, a chiral carbon is the one which has four different substituents attached to it.
- If the carbon is unsaturated then it is achiral only because during unsaturation, two or three bonds are formed with the same substituent.
- The structure of glucose molecule is,
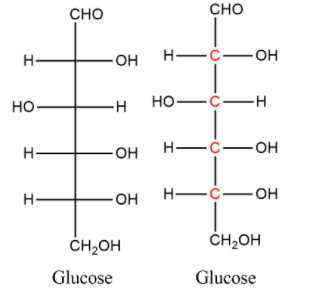
- Glucose has six carbon atoms. Out of that, first and last carbon are definitely achiral because one carbon is unsaturated and another one is forming two bonds with hydrogen atoms.
- The middle four carbon atoms in the chain are chiral because they all distinctly have four different substituents attached to them. They are shown in red ink above.
- Therefore, the number of chiral carbon atoms in glucose are 4.
-Therefore, the correct option is (A).
Note: Remember chiral carbon atoms are the carbon atoms which lack symmetry and have four different substituents attached to it. Chiral carbon is also known as asymmetric carbon. Enantiomers are pairs of compounds which are non-superimposable mirror images of each other. Achiral carbon is the carbon which is bonded to at least one same substituent or has unsaturation.
Complete step by step solution:
- Chirality is the property of asymmetry in a molecule or any substance.
- If the mirror image of a substance is non-superimposable with that substance then, that substance is said to possess chirality and the substance is said to be chiral or asymmetric. It is represented by an asterisk (*).
- For example the letter ‘A’ has the mirror image ‘A’ only so both the images can be superimposed as one image. So, the letter ‘A’ is not chiral or achiral.
- For letter ‘B’ or ‘D’ or ‘P’, its mirror images are non-superimposable to each other and therefore, these letters are chiral and enantiomers of each other.
- Another example is of hand, look at the palms of both of your hands. They are mirror images of each other. But when you keep your right hand palm on your left hand palm, like stacking, they do not superimpose on each other. You can see that at least both your thumbs are on different sides. Therefore, palms are chiral and they are enantiomers of each other.
- In chemistry, a chiral carbon is the one which has four different substituents attached to it.
- If the carbon is unsaturated then it is achiral only because during unsaturation, two or three bonds are formed with the same substituent.
- The structure of glucose molecule is,

- Glucose has six carbon atoms. Out of that, first and last carbon are definitely achiral because one carbon is unsaturated and another one is forming two bonds with hydrogen atoms.
- The middle four carbon atoms in the chain are chiral because they all distinctly have four different substituents attached to them. They are shown in red ink above.
- Therefore, the number of chiral carbon atoms in glucose are 4.
-Therefore, the correct option is (A).
Note: Remember chiral carbon atoms are the carbon atoms which lack symmetry and have four different substituents attached to it. Chiral carbon is also known as asymmetric carbon. Enantiomers are pairs of compounds which are non-superimposable mirror images of each other. Achiral carbon is the carbon which is bonded to at least one same substituent or has unsaturation.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE

In a human foetus the limbs and digits develop after class 12 biology CBSE

AABbCc genotype forms how many types of gametes a 4 class 12 biology CBSE

The correct structure of ethylenediaminetetraacetic class 12 chemistry CBSE




