
Chloretone is:
A. $CC{{l}_{3}}N{{O}_{2}}$
B. $C{{H}_{3}}COC{{H}_{2}}CHC{{l}_{2}}$
C. ${{\left( C{{H}_{3}} \right)}_{2}}COCHC{{l}_{2}}$
D. ${{\left( C{{H}_{3}} \right)}_{2}}C\left( OH \right)CC{{l}_{3}}$
Answer
573.6k+ views
Hint: Chloretone is going to form by the condensation of chloroform with acetone. Chloretone has a functional group of hydroxyl and contains three chlorine atoms in it. Chloretone is also called chlorobutanol.
Complete answer:
- Chloretone has antibacterial and antifungal properties.
- The molecular formula of chloretone is ${{C}_{4}}{{H}_{7}}C{{l}_{3}}O$ .
- Chlorine reacts with acetone and forms chloretone and the chemical reaction of chlorine with acetone is as follows.
\[\underset{Chloroform}{\mathop{CHC{{l}_{3}}}}\,+\underset{acetone~}{\mathop{C{{H}_{3}}-CO-C{{H}_{3}}}}\,\to \underset{Chloretone~}{\mathop{{{(C{{H}_{3}})}_{2}}C(OH)-CC{{l}_{3}}}}\,\]
- The structure of the chloretone is as follows.
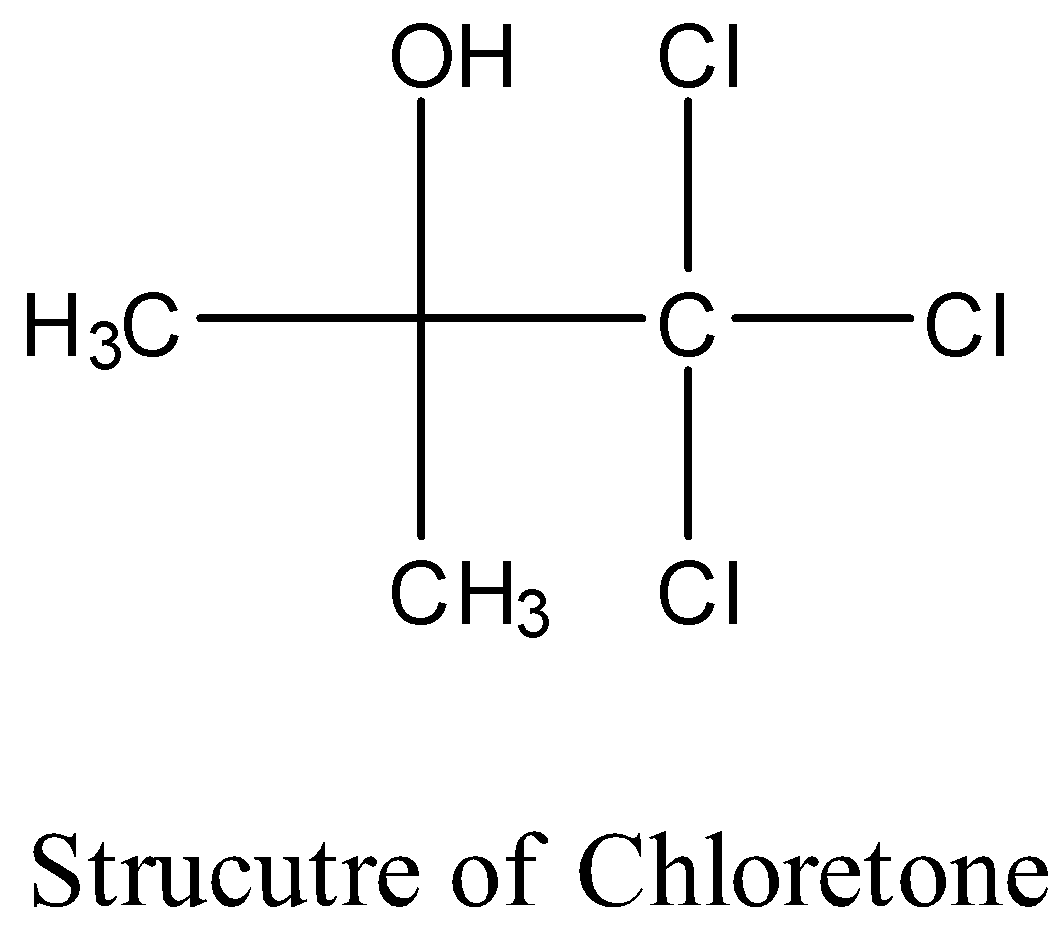
- Therefore the molecular formula of chloretone is ${{\left( C{{H}_{3}} \right)}_{2}}C\left( OH \right)CC{{l}_{3}}$ .
So, the correct option is D.
Additional information:
- The other name of chloretone is Hypnotic Chloretone. Very less amount (0.5 %) of chloretone is sufficient to kill the bacteria and fungus.
- The IUPAC name of chloretone is 1,1,1-trichloro-2-methyl-2-propanol.
- Chloretone is a white colored solid having a smell of methanol.
- Chloretone is formed by the nucleophilic addition of chloroform and acetone in the presence of a strong base.
- It is soluble in acetone and less soluble in water.
- It is sometimes used as a preservative with zero surfactant activity.
- After the preparation of chloretone from acetone and chloroform, the formed chloretone contains some impurities. The impurities have to be removed by a process called sublimation.
Note: The general name of chloretone is chlorobutanol. Chloretone is a white color volatile solid. It is highly toxic to the liver and it irritates the eye. It was first synthesized by Conrad Willgerodt in 1881.
Complete answer:
- Chloretone has antibacterial and antifungal properties.
- The molecular formula of chloretone is ${{C}_{4}}{{H}_{7}}C{{l}_{3}}O$ .
- Chlorine reacts with acetone and forms chloretone and the chemical reaction of chlorine with acetone is as follows.
\[\underset{Chloroform}{\mathop{CHC{{l}_{3}}}}\,+\underset{acetone~}{\mathop{C{{H}_{3}}-CO-C{{H}_{3}}}}\,\to \underset{Chloretone~}{\mathop{{{(C{{H}_{3}})}_{2}}C(OH)-CC{{l}_{3}}}}\,\]
- The structure of the chloretone is as follows.

- Therefore the molecular formula of chloretone is ${{\left( C{{H}_{3}} \right)}_{2}}C\left( OH \right)CC{{l}_{3}}$ .
So, the correct option is D.
Additional information:
- The other name of chloretone is Hypnotic Chloretone. Very less amount (0.5 %) of chloretone is sufficient to kill the bacteria and fungus.
- The IUPAC name of chloretone is 1,1,1-trichloro-2-methyl-2-propanol.
- Chloretone is a white colored solid having a smell of methanol.
- Chloretone is formed by the nucleophilic addition of chloroform and acetone in the presence of a strong base.
- It is soluble in acetone and less soluble in water.
- It is sometimes used as a preservative with zero surfactant activity.
- After the preparation of chloretone from acetone and chloroform, the formed chloretone contains some impurities. The impurities have to be removed by a process called sublimation.
Note: The general name of chloretone is chlorobutanol. Chloretone is a white color volatile solid. It is highly toxic to the liver and it irritates the eye. It was first synthesized by Conrad Willgerodt in 1881.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




