
Chlorination of toluene in presence of light and heat followed by treatment with aqueous $\text{NaOH}$ gives:
A. o-cresol
B. p-cresol
C. Mixture of o-cresol and p-cresol
D. Benzoic acid
Answer
590.4k+ views
Hint: It is a two-step conversion question, chlorination is free-radical mechanism of adding chlorine radicals to the methyl group of toluene and then, addition of aqueous $\text{NaOH}$ is like hydrolysis of the chlorine atoms attached to the carbon atom. The conversion is followed as${{\text{C}}_{6}}{{\text{H}}_{5}}\text{C}{{\text{H}}_{3}}\xrightarrow{\text{C}{{\text{l}}_{2}}/\text{hv}}\text{A}\xrightarrow{\text{aq}\text{. NaOH}}\text{B}$.
Complete step by step answer:
Let us do the conversion of the question,
A. Chlorination: In this, light and heat initiates the substitution process by breaking the chlorine molecule $\left( \text{C}{{\text{l}}_{2}} \right)$ into chlorine radicals $\left( \text{C}{{\text{l}}^{\bullet }} \right)$, which further breaks the alkane into radicals and proceeds the reaction forward. This reaction is shown by aliphatic hydrocarbon not by aromatic hydrocarbon. Here, aliphatic is the methyl group attached to benzene of toluene. The reaction replaces all the hydrogen atoms of methyl group $\left( -\text{C}{{\text{H}}_{3}} \right)$ by chlorine atoms $\left( -\text{CC}{{\text{l}}_{3}} \right)$. The reaction is
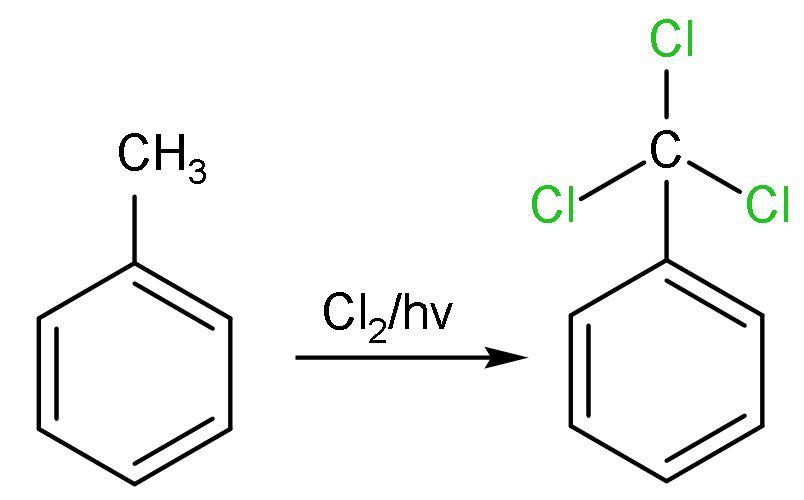
B. Hydrolysis: Hydrolysis is addition of water molecules. The chlorine atoms of $\left( -\text{CC}{{\text{l}}_{3}} \right)$ is replaced by the $\left( -\text{OH} \right)$ groups. All the three chlorine atoms are removed and $\left( -\text{OH} \right)$ group is added. $\left[ -\text{C}{{\left( \text{OH} \right)}_{3}} \right]$ has three $\left( -\text{OH} \right)$ groups, which is very unstable. It is unstable because of the steric hindrance and mainly due to interaction between $\left( -\text{OH} \right)$ groups which lessens the bond angle and repulsions occur. So, the $\left( -\text{OH} \right)$ groups are removed by releasing water and forming carbonyl groups. The reaction is
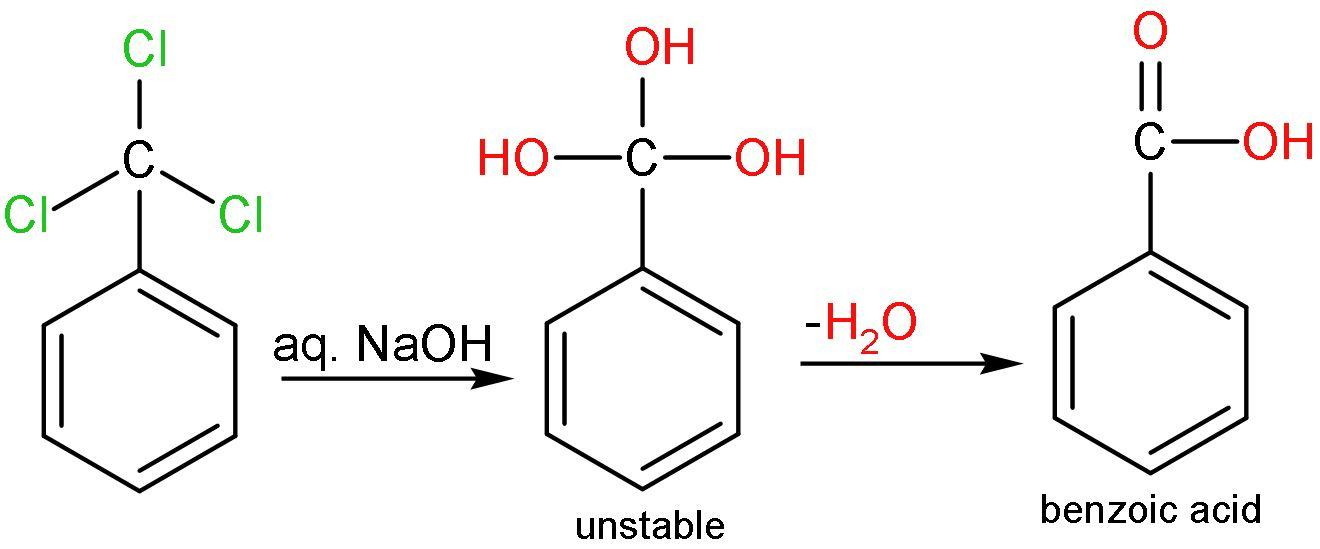
The compound formed will be Benzoic acid. So, the correct answer is “Option D”.
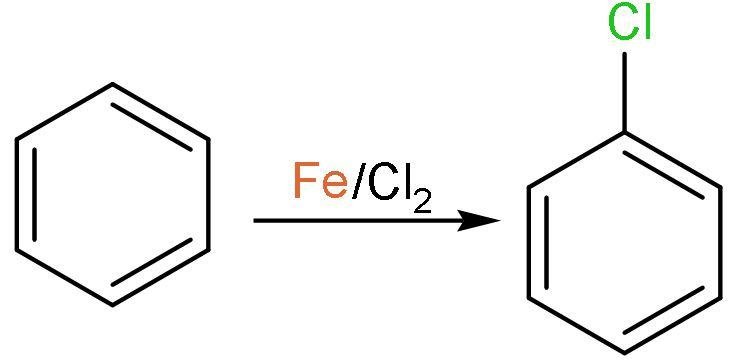
Note: The chlorination of aromatic compounds is done using the reagent and Lewis acid $\left( \text{FeC}{{\text{l}}_{3}} \right)$. The reaction is electrophilic substitution reaction. The compound formed chloro benzene $\left( {{\text{C}}_{6}}{{\text{H}}_{5}}\text{Cl} \right)$. The reaction is
Complete step by step answer:
Let us do the conversion of the question,
A. Chlorination: In this, light and heat initiates the substitution process by breaking the chlorine molecule $\left( \text{C}{{\text{l}}_{2}} \right)$ into chlorine radicals $\left( \text{C}{{\text{l}}^{\bullet }} \right)$, which further breaks the alkane into radicals and proceeds the reaction forward. This reaction is shown by aliphatic hydrocarbon not by aromatic hydrocarbon. Here, aliphatic is the methyl group attached to benzene of toluene. The reaction replaces all the hydrogen atoms of methyl group $\left( -\text{C}{{\text{H}}_{3}} \right)$ by chlorine atoms $\left( -\text{CC}{{\text{l}}_{3}} \right)$. The reaction is

B. Hydrolysis: Hydrolysis is addition of water molecules. The chlorine atoms of $\left( -\text{CC}{{\text{l}}_{3}} \right)$ is replaced by the $\left( -\text{OH} \right)$ groups. All the three chlorine atoms are removed and $\left( -\text{OH} \right)$ group is added. $\left[ -\text{C}{{\left( \text{OH} \right)}_{3}} \right]$ has three $\left( -\text{OH} \right)$ groups, which is very unstable. It is unstable because of the steric hindrance and mainly due to interaction between $\left( -\text{OH} \right)$ groups which lessens the bond angle and repulsions occur. So, the $\left( -\text{OH} \right)$ groups are removed by releasing water and forming carbonyl groups. The reaction is

The compound formed will be Benzoic acid. So, the correct answer is “Option D”.
Note: The chlorination of aromatic compounds is done using the reagent and Lewis acid $\left( \text{FeC}{{\text{l}}_{3}} \right)$. The reaction is electrophilic substitution reaction. The compound formed chloro benzene $\left( {{\text{C}}_{6}}{{\text{H}}_{5}}\text{Cl} \right)$. The reaction is

Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

What is a transformer Explain the principle construction class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE




