
Chlorobenzene is
A. less reactive than benzyl chloride
B. More reactive than ethyl bromide
C. Nearly as reactive as methyl chloride
D. More reactive than isopropyl chloride
Answer
554.1k+ views
Hint:This question is based on the reactivity of the compounds. We know that reactivity varies from one type of compound to that of another type, and depends on various factors. So, we need to solve this question option by option, by comparing the reactivity of chlorobenzene with different compounds mentioned in the options.
Complete step-by-step answer:Let us solve this question by comparing the reactivity of chlorobenzene with different compounds mentioned in the options. Let us start with option A.
A. Here we are given the compound benzyl chloride. so, firstly let us compare chlorobenzene and benzyl chloride.
In chlorobenzene, there are three lone pairs of electrons. These lone pairs on the chlorine undergo resonance with the $\pi $electrons of the benzene ring. Due to this resonance the C-Cl bond acquires a double bond character in it. And we know that double bonds are more strong and thus difficult to break. Hence, such compounds are not very reactive.
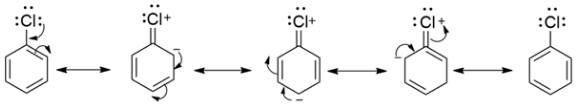
Whereas in benzyl chloride, the loss of chlorine yields a benzyl carbocation, which is resonance stabilized. Hence it is more reactive.
Therefore, chlorobenzene is less reactive than benzyl chloride. This option is correct.
Now let us move to option B.
B. the given compound is ethyl bromide. In this case also chlorobenzene will be less reactive than the ethyl bromide, due to the development of double bond character in the C-Cl bond. Therefore, we can say that chlorobenzene will be less reactive than ethyl bromide. This option, thus is not correct.
For the same reason that chlorobenzene acquires a double bond character in it through the resonance on lone pairs of electrons of chlorine, it will be less reactive than the other compounds which contain single bonds.
In option C and D, we are provided with methyl chloride and isopropyl chloride respectively. Both of these contain a single bond. Hence, they will be more reactive than chlorobenzene. Hence, the statements of these options are also wrong.
Therefore, from all the above explanations we can say that Option A. is the correct option.
Note: In organic compounds reactivity depends on various factors such as resonance, development of partial double bond, inductive effect, resonance, hyperconjugation etc. In organic reactions, reactivity predominantly depends on the stability of the intermediate form. If the intermediate is stable, the reaction will proceed and thus the substrates or reactants will be more stable.
Complete step-by-step answer:Let us solve this question by comparing the reactivity of chlorobenzene with different compounds mentioned in the options. Let us start with option A.
A. Here we are given the compound benzyl chloride. so, firstly let us compare chlorobenzene and benzyl chloride.
In chlorobenzene, there are three lone pairs of electrons. These lone pairs on the chlorine undergo resonance with the $\pi $electrons of the benzene ring. Due to this resonance the C-Cl bond acquires a double bond character in it. And we know that double bonds are more strong and thus difficult to break. Hence, such compounds are not very reactive.

Whereas in benzyl chloride, the loss of chlorine yields a benzyl carbocation, which is resonance stabilized. Hence it is more reactive.
Therefore, chlorobenzene is less reactive than benzyl chloride. This option is correct.
Now let us move to option B.
B. the given compound is ethyl bromide. In this case also chlorobenzene will be less reactive than the ethyl bromide, due to the development of double bond character in the C-Cl bond. Therefore, we can say that chlorobenzene will be less reactive than ethyl bromide. This option, thus is not correct.
For the same reason that chlorobenzene acquires a double bond character in it through the resonance on lone pairs of electrons of chlorine, it will be less reactive than the other compounds which contain single bonds.
In option C and D, we are provided with methyl chloride and isopropyl chloride respectively. Both of these contain a single bond. Hence, they will be more reactive than chlorobenzene. Hence, the statements of these options are also wrong.
Therefore, from all the above explanations we can say that Option A. is the correct option.
Note: In organic compounds reactivity depends on various factors such as resonance, development of partial double bond, inductive effect, resonance, hyperconjugation etc. In organic reactions, reactivity predominantly depends on the stability of the intermediate form. If the intermediate is stable, the reaction will proceed and thus the substrates or reactants will be more stable.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




