
Chlorobenzene on treatment with sodium in dry ether gives Diphenyl. The name of the reaction is _____.
(A) Fittig reaction
(B) Wurtz-Fittig reaction
(C) Sandmeyer reaction
(D) Gattermann reaction
Answer
594.3k+ views
Hint:
Coupling of two aryl groups in presence of sodium and ether is Fittig reaction. Coupling of one aryl group and one alkyl group in the same conditions is the Wurtz-Fittig reaction. In the Sandmeyer reaction, aromatic amines are used as starting materials and in Gattermann reactions, aldehydes are obtained as products.
Complete answer:
Here, the reaction that is given in the question.
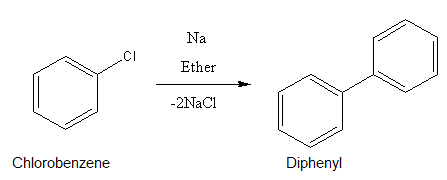
Whenever an aryl halide couples with itself in presence of Sodium metals and dry ether, the reaction is called Fittig reaction.
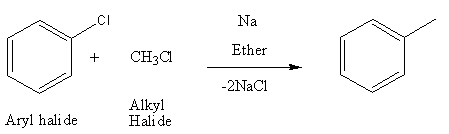
Now, Wurtz reaction has the same reagents as Fittig reaction but it differs in reactants used. In Wurtz fittig reaction, we use one alkyl halide and one aryl halide that couples to give product.
- Sandmayer reaction has aromatic amine as a reactant which is not here in this reaction. So, it is not a Sandmayer reaction.
- Gatterman reaction produces aldehydes, that is also not occurring here, so it is not Gattermann reaction.
As two aryl groups are combining to give diphenyl, it is a Fittig reaction.
So, correct answer is (A) Fittig reaction
Additional Information:
Example of Wurtz-Fittig reaction:
Example of Wurtz reaction :
\[\underset{alkylhalide}{\mathop{R-Cl}}\,+\underset{alkylhalide}{\mathop{R'-Cl}}\,\xrightarrow[Ether]{Na}R-R'+2NaCl\]
Note:
Be careful while choosing between Fittig, Wurtz-Fittig and Wurtz reaction because they have the same reagents and solvents used but they only differ in the starting materials they use.
Coupling of two aryl groups in presence of sodium and ether is Fittig reaction. Coupling of one aryl group and one alkyl group in the same conditions is the Wurtz-Fittig reaction. In the Sandmeyer reaction, aromatic amines are used as starting materials and in Gattermann reactions, aldehydes are obtained as products.
Complete answer:
Here, the reaction that is given in the question.

Whenever an aryl halide couples with itself in presence of Sodium metals and dry ether, the reaction is called Fittig reaction.
Now, Wurtz reaction has the same reagents as Fittig reaction but it differs in reactants used. In Wurtz fittig reaction, we use one alkyl halide and one aryl halide that couples to give product.
- Sandmayer reaction has aromatic amine as a reactant which is not here in this reaction. So, it is not a Sandmayer reaction.
- Gatterman reaction produces aldehydes, that is also not occurring here, so it is not Gattermann reaction.
As two aryl groups are combining to give diphenyl, it is a Fittig reaction.
So, correct answer is (A) Fittig reaction
Additional Information:
Example of Wurtz-Fittig reaction:

Example of Wurtz reaction :
\[\underset{alkylhalide}{\mathop{R-Cl}}\,+\underset{alkylhalide}{\mathop{R'-Cl}}\,\xrightarrow[Ether]{Na}R-R'+2NaCl\]
Note:
Be careful while choosing between Fittig, Wurtz-Fittig and Wurtz reaction because they have the same reagents and solvents used but they only differ in the starting materials they use.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




