
When chloroform is treated with oxygen, it forms:
(A) $COC{l_2} + HCl$
(B) $COC{l_2} + C{l_2} + {H_2}$
(C) $COC{l_2} + C{l_2} + {H_2}O$
(D) no product will be formed
Answer
580.5k+ views
Hint: Chloroform is halogen derivatives of alkane. Its formula is $CHC{l_3}$.
Chloroform is formed by replacing 3 H-atoms of methane by chlorine in presence of sunlight.
Its IUPAC name is Trichloromethane.
Complete answer:
Chloroform is colorless, volatile liquid and generally stored in dark colour bottles filled up to brim.
If it reacts with oxygen in presence of light it gives carbonyl chloride.
$CHC{l_3} + \dfrac{1}{2}{O_2}\xrightarrow{{sunlight}}COC{l_2} + HCl$
Carbonyl chloride is also known as phosgene gas.
To prevent formation of phosgene gas, chloroform should be stored in dark bottles with a small percentage of ethanol added.
Therefore, from the above explanation the correct option is (A) $COC{l_2} + HCl.$
Additional Information: Chloroform is colorless, volatile liquid and has ether like odor. Formally it is used as an inhaled anesthetic surgery.
Today it is used as solvent in industry, where used in production of refrigerant from.
Chloroform is denser than water and slightly soluble in water. Hence, sink in water.
It may cause illness by inhalation, skin absorption or ingestion. Chloroform adopts tetrahedral geometry.
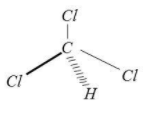
Note: Oxidation of chloroform is a dangerous reaction. It releases poisonous gas.
In the past chloroform was used as an inhaled anesthetic drug during surgery but it is not used that day. Today it is used to make other chemicals.
Chloroform is formed by replacing 3 H-atoms of methane by chlorine in presence of sunlight.
Its IUPAC name is Trichloromethane.
Complete answer:
Chloroform is colorless, volatile liquid and generally stored in dark colour bottles filled up to brim.
If it reacts with oxygen in presence of light it gives carbonyl chloride.
$CHC{l_3} + \dfrac{1}{2}{O_2}\xrightarrow{{sunlight}}COC{l_2} + HCl$
Carbonyl chloride is also known as phosgene gas.
To prevent formation of phosgene gas, chloroform should be stored in dark bottles with a small percentage of ethanol added.
Therefore, from the above explanation the correct option is (A) $COC{l_2} + HCl.$
Additional Information: Chloroform is colorless, volatile liquid and has ether like odor. Formally it is used as an inhaled anesthetic surgery.
Today it is used as solvent in industry, where used in production of refrigerant from.
Chloroform is denser than water and slightly soluble in water. Hence, sink in water.
It may cause illness by inhalation, skin absorption or ingestion. Chloroform adopts tetrahedral geometry.

Note: Oxidation of chloroform is a dangerous reaction. It releases poisonous gas.
In the past chloroform was used as an inhaled anesthetic drug during surgery but it is not used that day. Today it is used to make other chemicals.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




