
Choose the correct option for the following:
Assertion:$NC{l_3}$ undergoes hydrolysis
Reason:Chlorine has vacant $d - $ orbital
(A) Both assertion and reason are correct. The reason is the correct explanation for the assertion.
(B) Both assertion and reason are correct. The reason is not the correct explanation for the assertion.
(C) The assertion is correct but the reason is incorrect.
(D) Both assertion and reason are incorrect.
Answer
564.9k+ views
Hint:We know that hydrolysis means ‘reaction with water’, so hydrolysis of nitrogen trichloride means the reaction of nitrogen trichloride with water. Nitrogen trichloride has a molecular formula $NC{l_3}$ . It is a nonpolar covalent compound.
Complete answer:We know that Nitrogen trichloride has a molecular formula $NC{l_3}$ . It is also called trichloramine. Nitrogen trichloride is obtained when ammonium salts like ammonium nitrate react with chlorine. We can write the structure of $NC{l_3}$ as
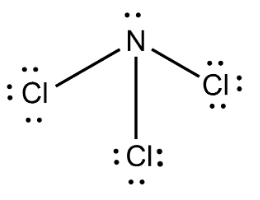
Here nitrogen and chlorine form three covalent bonds. $N$ has an atomic number $7$ and it has an electronic configuration $1{s^2}2{s^2}2{p^3}$ , we can see that $p - $ orbital is half-filled. Chlorine has an atomic number $17$ . The electronic configuration of chlorine can be written as $1{s^2}2{s^2}2{p^6}3{s^2}3{p^5}$ .
Now according to our question, the assertion is $NC{l_3}$ undergoes hydrolysis. This means we have to react $NC{l_3}$ with water. Hydrolysis of nitrogen trichloride is done by hot water. The following reaction will occur:
$NC{l_3} + 3{H_2}O \to N{H_3} + 3HOCl$
This means the assertion is correct. The products formed on hydrolysis of $NC{l_3}$ are ammonia and hypochlorous acid.
From the above diagrams, we see that nitrogen needs three electrons to complete $p - $ orbital and thus forms three covalent bonds with chlorine atoms. Now the $p - $ orbital of nitrogen is completely filled and therefore water molecules cannot attack nitrogen to give lone pairs of electrons. Chlorine has vacant $d - $ orbitals and thus water molecules attack chlorine. Thus the given reason is also correct.
So, the correct option is $(A)$.
Note:We should also know that $NC{l_3}$ is an unstable compound. This is because the sizes of nitrogen and chlorine are different and also the bonding between $2p$ of nitrogen and $3p$ of chlorine is not strong enough. Therefore $NC{l_3}$ is unstable and explosive.
Complete answer:We know that Nitrogen trichloride has a molecular formula $NC{l_3}$ . It is also called trichloramine. Nitrogen trichloride is obtained when ammonium salts like ammonium nitrate react with chlorine. We can write the structure of $NC{l_3}$ as

Here nitrogen and chlorine form three covalent bonds. $N$ has an atomic number $7$ and it has an electronic configuration $1{s^2}2{s^2}2{p^3}$ , we can see that $p - $ orbital is half-filled. Chlorine has an atomic number $17$ . The electronic configuration of chlorine can be written as $1{s^2}2{s^2}2{p^6}3{s^2}3{p^5}$ .
Now according to our question, the assertion is $NC{l_3}$ undergoes hydrolysis. This means we have to react $NC{l_3}$ with water. Hydrolysis of nitrogen trichloride is done by hot water. The following reaction will occur:
$NC{l_3} + 3{H_2}O \to N{H_3} + 3HOCl$
This means the assertion is correct. The products formed on hydrolysis of $NC{l_3}$ are ammonia and hypochlorous acid.
From the above diagrams, we see that nitrogen needs three electrons to complete $p - $ orbital and thus forms three covalent bonds with chlorine atoms. Now the $p - $ orbital of nitrogen is completely filled and therefore water molecules cannot attack nitrogen to give lone pairs of electrons. Chlorine has vacant $d - $ orbitals and thus water molecules attack chlorine. Thus the given reason is also correct.
So, the correct option is $(A)$.
Note:We should also know that $NC{l_3}$ is an unstable compound. This is because the sizes of nitrogen and chlorine are different and also the bonding between $2p$ of nitrogen and $3p$ of chlorine is not strong enough. Therefore $NC{l_3}$ is unstable and explosive.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




