
Choose the correct option for - which is not the structure of $ {C_4}{H_9}Br $
(A) $ 1 - $ Bromobutane
(B) $ 2 - $ Bromobutane
(C) $ 1 - $ Bromo - $ 2 - $ methylpropane
(D) Isobutane
Answer
521.1k+ views
Hint :Bromobutane is a colourless liquid, but its impure sample can be seen as slightly yellow in colour. Bromobutane is an organobromine compound with a molecular formula of $ {C_4}{H_9}Br $ and the boiling point of around $ 100 - {205^o}C $ .
Complete Step By Step Answer:
To solve this question we should have some insight regarding the conformation of structures.
Looking at our options - $ 1 - $ Bromobutane and $ 2 - $ Bromobutane, let’s have a look at their structure for the better understanding –
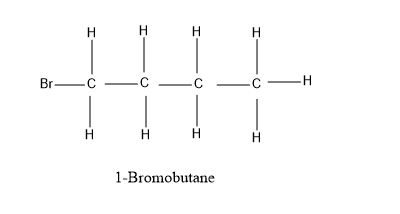
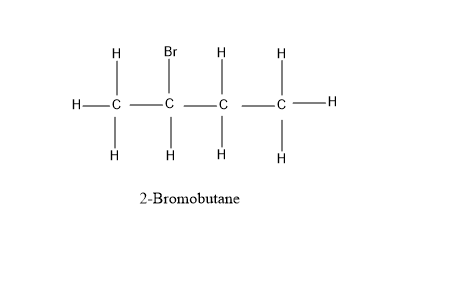
As, it is evident from the diagram above, that $ 1 - $ Bromobutane and $ 2 - $ Bromobutane fits completely into the formula $ {C_4}{H_9}Br $ . All the valencies of all the atoms are completely satisfied and have a stable structure under this formula - $ {C_4}{H_9}Br $ .
Similarly. $ 1 - $ Bromo - $ 2 - $ methyl propane – also has a stable structure satisfying all the valences of each atom.
But, coming to our option D , it is isobutane . It has a molecular formula of $ {C_4}{H_{10}} $ . Isobutane does not consist of bromine atoms. So it does not fit in our given formula $ {C_4}{H_9}Br $ .
So, the correct option to our question is D i.e. isobutane.
Note :
For $ {C_4}{H_9}Br $ we have four possible structural isomers and they are - $ 1 - $ Bromobutane and $ 2 - $ Bromobutane, $ 1 - $ Bromo - $ 2 - $ methylpropane, these three we just read above, but the fourth isomer that is possible is – tert-butyl bromide or $ 2 - $ Bromo - $ 2 - $ methylpropane. These bromobutane are less soluble in water and are denser than water.
Complete Step By Step Answer:
To solve this question we should have some insight regarding the conformation of structures.
Looking at our options - $ 1 - $ Bromobutane and $ 2 - $ Bromobutane, let’s have a look at their structure for the better understanding –


As, it is evident from the diagram above, that $ 1 - $ Bromobutane and $ 2 - $ Bromobutane fits completely into the formula $ {C_4}{H_9}Br $ . All the valencies of all the atoms are completely satisfied and have a stable structure under this formula - $ {C_4}{H_9}Br $ .
Similarly. $ 1 - $ Bromo - $ 2 - $ methyl propane – also has a stable structure satisfying all the valences of each atom.
But, coming to our option D , it is isobutane . It has a molecular formula of $ {C_4}{H_{10}} $ . Isobutane does not consist of bromine atoms. So it does not fit in our given formula $ {C_4}{H_9}Br $ .
So, the correct option to our question is D i.e. isobutane.
Note :
For $ {C_4}{H_9}Br $ we have four possible structural isomers and they are - $ 1 - $ Bromobutane and $ 2 - $ Bromobutane, $ 1 - $ Bromo - $ 2 - $ methylpropane, these three we just read above, but the fourth isomer that is possible is – tert-butyl bromide or $ 2 - $ Bromo - $ 2 - $ methylpropane. These bromobutane are less soluble in water and are denser than water.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




