
Choose the correct structure for propane.
A. $C{H_3} - C{H_2} - C{H_3}$
B.
C. $C{H_3} - O - {C_3}{H_7}$
D.
E. $C{H_3} - C{H_2} - N{H_2}$


Answer
558.9k+ views
Hint: Propane is an organic compound which belongs to the alkane family. It is also referred to as hydrocarbon which is composed only of carbon and hydrogen.
Complete step by step answer:
Alkane is a class of organic compounds which consists of acyclic saturated hydrocarbons. The alkanes are generally represented by the chemical formula \[{C_n}{H_{2n + 2}}\] . The term saturated means there is no unsaturation in the form of double or triple bonds. They only contain single bonds between the carbon atoms.
Methane is the simplest alkane of this family with one carbon atom. Propane is the compound with three carbon atoms. The carbon atoms in propane are bonded with single bonds between the three carbon atoms.
According to IUPAC the alkane is named with the number of carbon atoms first followed by the suffix –ane. Let us find names of the given structures based on the concept of IUPAC.
A. $C{H_3} - C{H_2} - C{H_3}$
In this molecule there are three carbon atoms which are bonded by single bonds. The chemical formula of the molecule is $C_3H_8$ which satisfies the formula of alkanes. Thus the name of the molecule is propane.
B.
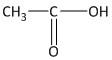
In this molecule a carboxylic functional group is attached to a methyl group. The total number of carbon atoms is two. Thus the name of the molecule is ethanoic acid or acetic acid.
C. $C{H_3} - O - {C_3}{H_7}$
In this molecule two propyl chains are flanked by one oxygen atom. Such a type of molecule is called an ether in organic chemistry. The name of the molecule is di n-propyl ether.
D.
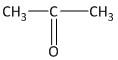
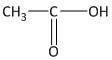
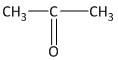
In this molecule a carbonyl functional group is present between two methyl groups. The compound contains a total of three carbon atoms of which the middle carbon is a carbonyl carbon. Thus the mane of the compound is propanone.
E. $C{H_3} - C{H_2} - N{H_2}$
In this molecule an amine functional group which is primary is attached to an ethyl group. Thus the name of the compound is ethyl amine.
Note:
The structure of a molecule is a two dimensional or three dimensional representation of the bonds between the bonded atoms. The bonds are of three types single, double or triple in nature.
Complete step by step answer:
Alkane is a class of organic compounds which consists of acyclic saturated hydrocarbons. The alkanes are generally represented by the chemical formula \[{C_n}{H_{2n + 2}}\] . The term saturated means there is no unsaturation in the form of double or triple bonds. They only contain single bonds between the carbon atoms.
Methane is the simplest alkane of this family with one carbon atom. Propane is the compound with three carbon atoms. The carbon atoms in propane are bonded with single bonds between the three carbon atoms.
According to IUPAC the alkane is named with the number of carbon atoms first followed by the suffix –ane. Let us find names of the given structures based on the concept of IUPAC.
A. $C{H_3} - C{H_2} - C{H_3}$
In this molecule there are three carbon atoms which are bonded by single bonds. The chemical formula of the molecule is $C_3H_8$ which satisfies the formula of alkanes. Thus the name of the molecule is propane.
B.

In this molecule a carboxylic functional group is attached to a methyl group. The total number of carbon atoms is two. Thus the name of the molecule is ethanoic acid or acetic acid.
C. $C{H_3} - O - {C_3}{H_7}$
In this molecule two propyl chains are flanked by one oxygen atom. Such a type of molecule is called an ether in organic chemistry. The name of the molecule is di n-propyl ether.
D.

In this molecule a carbonyl functional group is present between two methyl groups. The compound contains a total of three carbon atoms of which the middle carbon is a carbonyl carbon. Thus the mane of the compound is propanone.
E. $C{H_3} - C{H_2} - N{H_2}$
In this molecule an amine functional group which is primary is attached to an ethyl group. Thus the name of the compound is ethyl amine.
Note:
The structure of a molecule is a two dimensional or three dimensional representation of the bonds between the bonded atoms. The bonds are of three types single, double or triple in nature.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




