
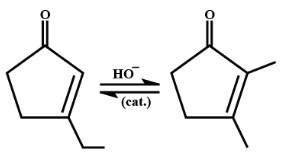
Choose the most reasonable reaction intermediate for the following reaction:
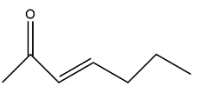
A.
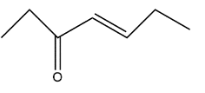
B.
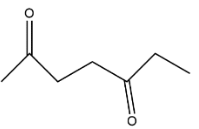
C.
D. None of these




Answer
541.8k+ views
Hint: The reaction given above generally based on the concept of aldol condensation which occurs in aldehydes and the necessary condition for aldol condensation is presence of $\alpha $ hydrogens with a dilute base which gives $\beta $ hydroxy aldehydes known by the name aldols.
Complete step-by-step answer:
In aldol condensation enolate ions react with a carbonyl compound to form $\beta $- hydroxy ketones or $\beta $- hydroxy aldehydes then dehydration occurs which give conjugated enone.
To choose the most stable intermediate for the given reaction we have to follow the following steps:
1. Break the bond between$\alpha $and $\beta $ carbon and attack of $O{{H}^{-}}$$O{{H}^{-}}$ion on them which can be shown as follows:
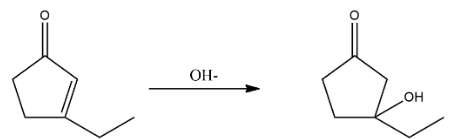
2. After the attacking of hydroxide ion retro aldol condensation takes place which opens the ring and the structure will be shown as:
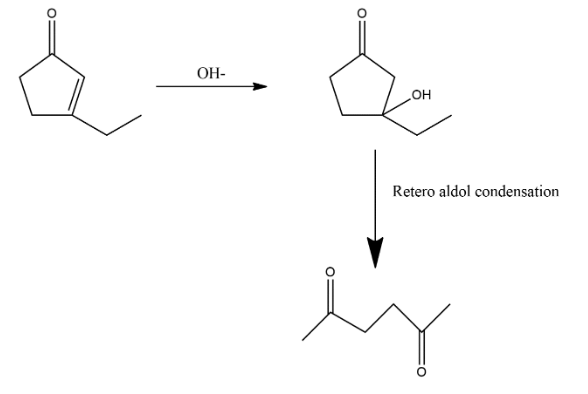
3. After this step again condensation process is done which again reform it in ring shape which can be shown as:
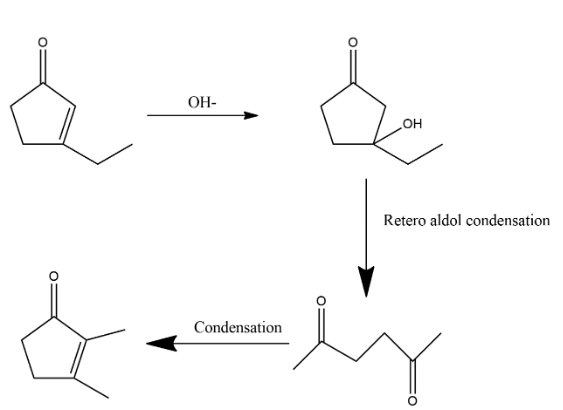
Hence by observing the whole reaction we can consider that the option C is the correct answer, this is the intermediate in the given reaction.
Note: Aldol condensations play a very important role in organic synthesis as it creates a path to form carbon-carbon bonds. When the same type of reaction occurs with two different types of carbonyl compounds then this type of reactions are known by the name cross aldol condensation.
Complete step-by-step answer:
In aldol condensation enolate ions react with a carbonyl compound to form $\beta $- hydroxy ketones or $\beta $- hydroxy aldehydes then dehydration occurs which give conjugated enone.
To choose the most stable intermediate for the given reaction we have to follow the following steps:
1. Break the bond between$\alpha $and $\beta $ carbon and attack of $O{{H}^{-}}$$O{{H}^{-}}$ion on them which can be shown as follows:

2. After the attacking of hydroxide ion retro aldol condensation takes place which opens the ring and the structure will be shown as:

3. After this step again condensation process is done which again reform it in ring shape which can be shown as:

Hence by observing the whole reaction we can consider that the option C is the correct answer, this is the intermediate in the given reaction.
Note: Aldol condensations play a very important role in organic synthesis as it creates a path to form carbon-carbon bonds. When the same type of reaction occurs with two different types of carbonyl compounds then this type of reactions are known by the name cross aldol condensation.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE

Give 10 examples of unisexual and bisexual flowers




