
Clemmensen reduction of aldehydes and ketones produce:
A.Alkenes
B.Tertiary alcohols
C.Alkanes
D.Primary alkanes
Answer
515.4k+ views
Hint: Clemmensen reduction is a chemical reaction in which ketones (or aldehydes) are converted to alkanes with the aid of zinc amalgam and distilled hydrochloric acid. Erik Christian Clemmensen, a Danish chemist, was the inspiration for this reaction.
Complete answer:
The Clemmensen Reduction enables aldehydes or ketones to be deoxygenated and the resulting hydrocarbon to be generated. Clemmensen reduction is a chemical reaction in which ketones (or aldehydes) are converted to alkanes with the aid of zinc amalgam and distilled hydrochloric acid. Erik Christian Clemmensen, a Danish chemist, was the inspiration for this reaction.
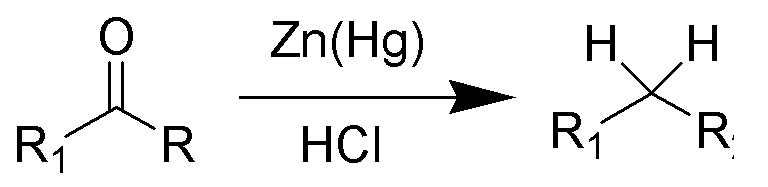
Aryl-alkyl ketones, such as those produced in a Friedel-Crafts acylation, react well to the initial Clemmensen reduction conditions. A classic technique for primary alkylation of arenes is a two-step sequence of Friedel-Crafts acylation followed by Clemmensen reduction. Changed Clemmensen conditions using active zinc dust in an anhydrous solution of hydrogen chloride in diethyl ether or acetic anhydride are much more successful for aliphatic or cyclic ketones.
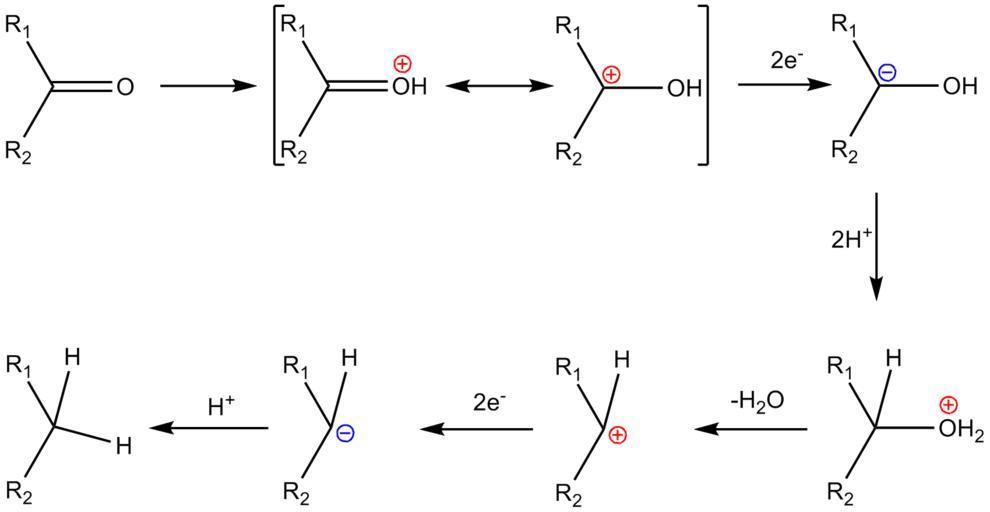
The substrate must be tolerant of the Clemmensen reduction's highly acidic conditions (37 percent HCl). There are several options available. The Wolff-Kishner reduction can be used on acid-sensitive substrates that are stable to heavy base; the two-step Mozingo reduction is a milder process for substrates that are stable to hydrogenolysis in the presence of Raney nickel. Despite the reaction's long history, the mechanism of the Clemmensen reduction is unknown. Mechanistic experiments are complicated due to the heterogeneous nature of the reaction, and only a few have been published.
And hence the correct answer is option C.
Note:
Organozinc intermediates, often including zinc carbenoids, are frequently mentioned in mechanistic formulations, either as distinct species or as organic fragments attached to the zinc metal surface. The resulting alcohol, on the other hand, is not thought to be an intermediate because it does not yield the alkane element when exposed to Clemmensen conditions.
Complete answer:
The Clemmensen Reduction enables aldehydes or ketones to be deoxygenated and the resulting hydrocarbon to be generated. Clemmensen reduction is a chemical reaction in which ketones (or aldehydes) are converted to alkanes with the aid of zinc amalgam and distilled hydrochloric acid. Erik Christian Clemmensen, a Danish chemist, was the inspiration for this reaction.

Aryl-alkyl ketones, such as those produced in a Friedel-Crafts acylation, react well to the initial Clemmensen reduction conditions. A classic technique for primary alkylation of arenes is a two-step sequence of Friedel-Crafts acylation followed by Clemmensen reduction. Changed Clemmensen conditions using active zinc dust in an anhydrous solution of hydrogen chloride in diethyl ether or acetic anhydride are much more successful for aliphatic or cyclic ketones.
The substrate must be tolerant of the Clemmensen reduction's highly acidic conditions (37 percent HCl). There are several options available. The Wolff-Kishner reduction can be used on acid-sensitive substrates that are stable to heavy base; the two-step Mozingo reduction is a milder process for substrates that are stable to hydrogenolysis in the presence of Raney nickel. Despite the reaction's long history, the mechanism of the Clemmensen reduction is unknown. Mechanistic experiments are complicated due to the heterogeneous nature of the reaction, and only a few have been published.

And hence the correct answer is option C.
Note:
Organozinc intermediates, often including zinc carbenoids, are frequently mentioned in mechanistic formulations, either as distinct species or as organic fragments attached to the zinc metal surface. The resulting alcohol, on the other hand, is not thought to be an intermediate because it does not yield the alkane element when exposed to Clemmensen conditions.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




