
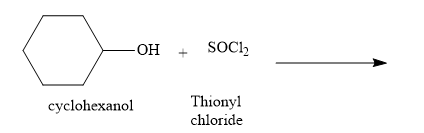
Complete the equation for the following reaction:

Answer
569.4k+ views
Hint: Identify the type of reaction such as substitution or elimination. Identify the leaving group and nucleophile present if any.
Complete answer:
Cyclohexanol is an alcohol. It reacts with thionyl chloride to form chlorocyclohexane. The byproducts are hydrogen chloride and sulphur dioxide. The byproducts are gaseous and are easily removed during the reaction. Due to this, the work up of this reaction is very easy. There is no difficulty in the isolation of the product from the reaction mixture. Hence, this method is the preferred method for the conversion of alcohols to alkyl chlorides.
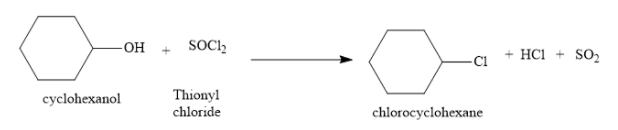
In the above reaction, an alcohol is converted into an alkyl chloride. Hydroxyl group is the leaving group and the chloride ion is the nucleophile. In this reaction, the bond between carbon atom and oxygen atom (of hydroxyl group) is broken. A new bond is formed between carbon atom and chlorine atom.
Of two chlorine atoms of thionyl chloride, one chlorine atom is a part of chlorocyclohexane and the other chlorine atom is part of hydrogen chloride. The oxygen atom of the hydroxyl group of alcohol is the part of sulphur dioxide molecule.
Note:
You can also use phosphorus trichloride or phosphorous pentachloride to convert an alcohol to alkyl halide. You can also carry out a nucleophilic substitution reaction of an alcohol with hydrogen chloride to obtain alkyl chloride.
Complete answer:
Cyclohexanol is an alcohol. It reacts with thionyl chloride to form chlorocyclohexane. The byproducts are hydrogen chloride and sulphur dioxide. The byproducts are gaseous and are easily removed during the reaction. Due to this, the work up of this reaction is very easy. There is no difficulty in the isolation of the product from the reaction mixture. Hence, this method is the preferred method for the conversion of alcohols to alkyl chlorides.

In the above reaction, an alcohol is converted into an alkyl chloride. Hydroxyl group is the leaving group and the chloride ion is the nucleophile. In this reaction, the bond between carbon atom and oxygen atom (of hydroxyl group) is broken. A new bond is formed between carbon atom and chlorine atom.
Of two chlorine atoms of thionyl chloride, one chlorine atom is a part of chlorocyclohexane and the other chlorine atom is part of hydrogen chloride. The oxygen atom of the hydroxyl group of alcohol is the part of sulphur dioxide molecule.
Note:
You can also use phosphorus trichloride or phosphorous pentachloride to convert an alcohol to alkyl halide. You can also carry out a nucleophilic substitution reaction of an alcohol with hydrogen chloride to obtain alkyl chloride.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




