
Complete the following chemical reaction.
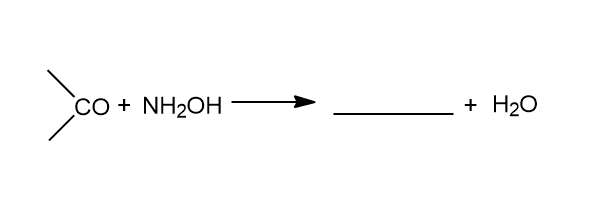

Answer
512.4k+ views
Hint: The above reaction is very simple. Here carbonyl is reacting with \[N{H_2}OH\] which is known as hydroxylamine to yield the product. The product formed is called oxime. The carbonyl here reacting can be an aldehyde or a ketone.
Complete answer:
We are given above with an incomplete reaction, and it is asked to complete the reaction. The incomplete reaction given is:
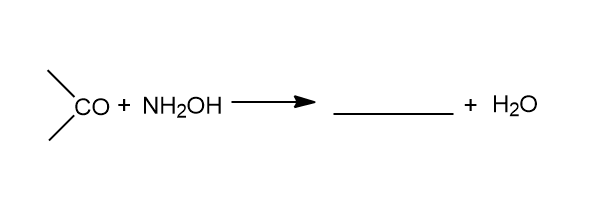
In the reaction the carbonyl is reacting with \[N{H_2}OH\] which is also known as Hydroxylamine to yield the product. The carbonyl can be of an aldehyde or a ketone. This reaction is only possible with an aldehyde or a ketone molecule. During the reaction there is release of water molecules.
The reaction taking place above will give oxime as the product. And the reaction will take place as follows:
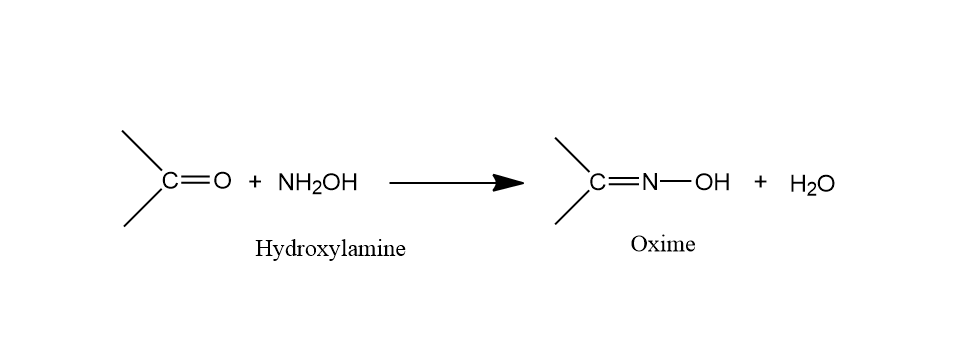
In the above reaction a carbonyl compound may be an aldehyde or a ketone is reacting with \[N{H_2}OH\] called hydroxylamine and there is release of water molecule to form the product oxime. When an aldehyde reacts with hydroxylamine then the product is called aldoxime and when the ketone reacts with the hydroxylamine then the product is called ketoxime. The product formed is called oxime. Oxime belongs to the class of compounds known as imines. The general formula of oximes is $R{R’}C = NOH$. Oximes can also be derived from the reaction of hydrogen donating reagents.
Note:
Oximes have applications in many areas. It is used as an antidote for nerve agents. In industries, caprolactum is an organic compound produced by the use of oxime, for nylon production caprolactam is used as a precursor. Oxime is also known as hydrazine.
Complete answer:
We are given above with an incomplete reaction, and it is asked to complete the reaction. The incomplete reaction given is:

In the reaction the carbonyl is reacting with \[N{H_2}OH\] which is also known as Hydroxylamine to yield the product. The carbonyl can be of an aldehyde or a ketone. This reaction is only possible with an aldehyde or a ketone molecule. During the reaction there is release of water molecules.
The reaction taking place above will give oxime as the product. And the reaction will take place as follows:

In the above reaction a carbonyl compound may be an aldehyde or a ketone is reacting with \[N{H_2}OH\] called hydroxylamine and there is release of water molecule to form the product oxime. When an aldehyde reacts with hydroxylamine then the product is called aldoxime and when the ketone reacts with the hydroxylamine then the product is called ketoxime. The product formed is called oxime. Oxime belongs to the class of compounds known as imines. The general formula of oximes is $R{R’}C = NOH$. Oximes can also be derived from the reaction of hydrogen donating reagents.
Note:
Oximes have applications in many areas. It is used as an antidote for nerve agents. In industries, caprolactum is an organic compound produced by the use of oxime, for nylon production caprolactam is used as a precursor. Oxime is also known as hydrazine.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




