
Complete the reaction:
${\text{N}}{{\text{H}}_4}{\text{CNO }}\xrightarrow{{{\text{heat}}}}{\text{ ?}}$
Answer
573.9k+ views
Hint: In order to complete a reaction, we must first identify the basic ions or functional groups present in the compound and think of all the possible ways in which it can react to form suitable products. In all reactions which involve heating, mostly two products may be formed, where in the initial product on product on prolonged heating yields another new product.
Complete step by step solution:
The chemical name of ${\text{N}}{{\text{H}}_4}{\text{CNO}}$ is ammonium cyanate. It is a colourless inorganic compound with the structure given below.
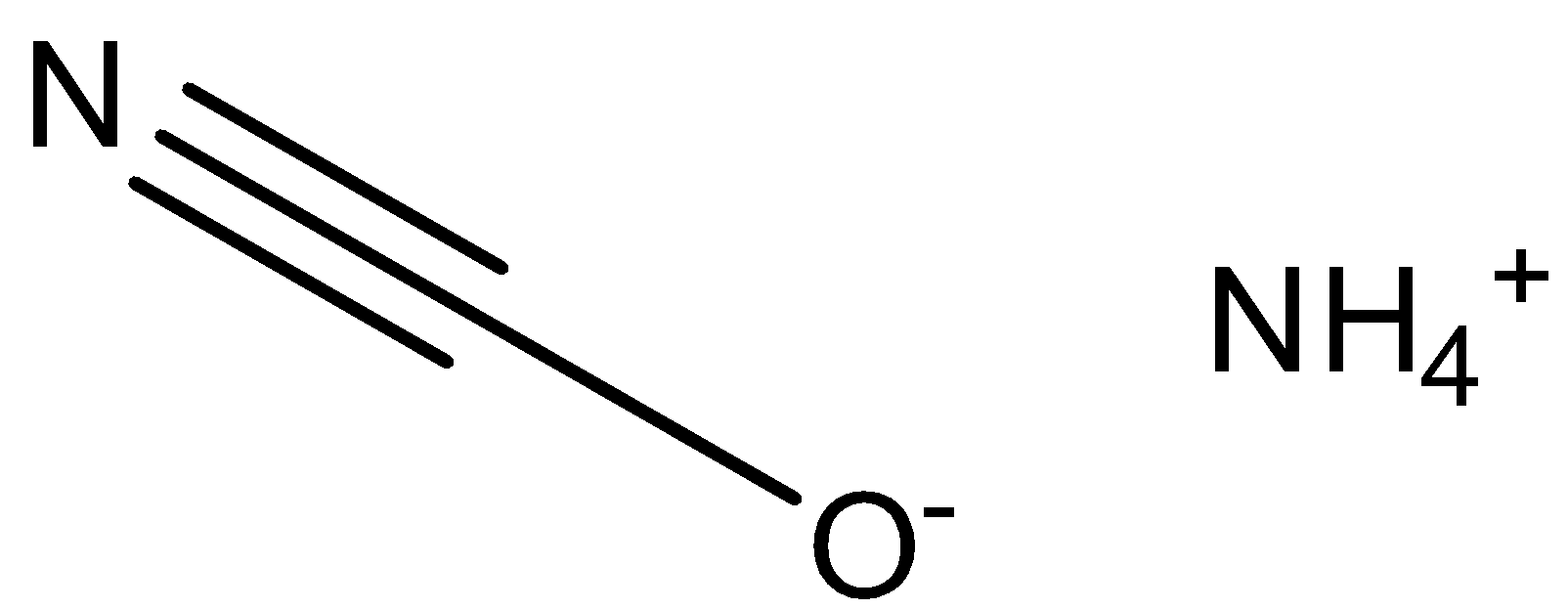
In order to draw the chemical structure of ammonium cyanate, we must first identify the individual constituents and their numbers present in the compound. Ammonium cyanate is a compound in which ammonium ion is attached with cyanate ion with a positive and negative charge respectively on the two ions present.
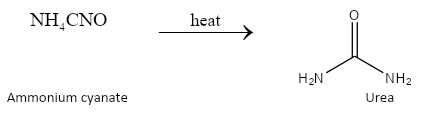
Ammonium cyanate on heating gives the compound known as urea. On prolonged heating of urea, a new product called biuret is formed.
The reactions can be depicted as follows:
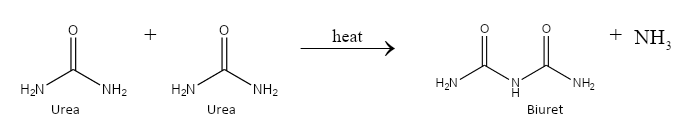
On prolonged heating, 2 molecules of urea combine together to produce biuret. We can depict the chemical reaction as follows:
Hence, the correct answer is urea.
Additional Information:
Ammonium cyanate is an inorganic compound used as a precursor for the synthesis of urea through Wohler synthesis of urea, which is an organic compound.
Note:
Remember that ammonium cyanate is an inorganic compound and the product formed on heating ammonium cyanate is urea. The prolonged heating of urea leads to a product called biuret. Biuret test is a test which is performed to determine the presence of peptide bonds.
Complete step by step solution:
The chemical name of ${\text{N}}{{\text{H}}_4}{\text{CNO}}$ is ammonium cyanate. It is a colourless inorganic compound with the structure given below.

In order to draw the chemical structure of ammonium cyanate, we must first identify the individual constituents and their numbers present in the compound. Ammonium cyanate is a compound in which ammonium ion is attached with cyanate ion with a positive and negative charge respectively on the two ions present.
Ammonium cyanate on heating gives the compound known as urea. On prolonged heating of urea, a new product called biuret is formed.
The reactions can be depicted as follows:

On prolonged heating, 2 molecules of urea combine together to produce biuret. We can depict the chemical reaction as follows:

Hence, the correct answer is urea.
Additional Information:
Ammonium cyanate is an inorganic compound used as a precursor for the synthesis of urea through Wohler synthesis of urea, which is an organic compound.
Note:
Remember that ammonium cyanate is an inorganic compound and the product formed on heating ammonium cyanate is urea. The prolonged heating of urea leads to a product called biuret. Biuret test is a test which is performed to determine the presence of peptide bonds.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




