
Compound A, ${{\text{C}}_{\text{8}}}{{\text{H}}_{{\text{10}}}}{\text{O}}$ is found to react with ${\text{NaOI}}$ (produced by reacting Y with ${\text{NaOH}}$) and yields a yellow precipitate with characteristic smell. A and Y are respectively.
A.
B.
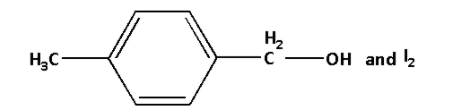
C.
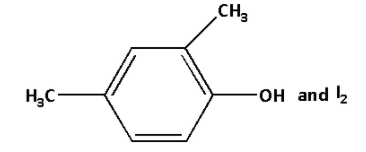
D.
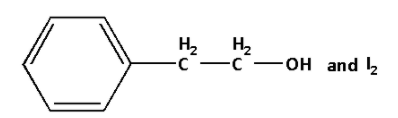




Answer
576.6k+ views
Hint: We are given that ${\text{NaOI}}$ is produced by the reaction of Y with ${\text{NaOH}}$. And compound A $\left( {{{\text{C}}_{\text{8}}}{{\text{H}}_{{\text{10}}}}{\text{O}}} \right)$ reacts with ${\text{NaOI}}$ produces a yellow precipitate. We must know the compound that forms yellow precipitate in the reaction.
Complete step by step solution:
First we will determine the compound Y as follows:
We are given that sodium hypoiodite $\left( {{\text{NaOI}}} \right)$ is produced by the reaction of Y with sodium hydroxide $\left( {{\text{NaOH}}} \right)$. ${\text{NaOI}}$ is produced when ${\text{NaOH}}$ reacts with ${{\text{I}}_2}$. The reaction is as follows:
${\text{NaOH}} + {{\text{I}}_2} \to {\text{NaOI}} + {\text{HI}}$
Thus, compound Y is ${{\text{I}}_2}$.
We are given that compound A $\left( {{{\text{C}}_{\text{8}}}{{\text{H}}_{{\text{10}}}}{\text{O}}} \right)$ reacts with ${\text{NaOI}}$ produces a yellow precipitate.
The yellow precipitate formed in the reaction is of iodoform. The molecular formula of iodoform is ${\text{C}}{{\text{H}}_{\text{3}}}{\text{I}}$.
We know that the iodoform test is positive for methyl ketones. Thus, the compound A must have methyl $\left( { - {\text{C}}{{\text{H}}_3}} \right)$ group and a ketonic group $\left( {{\text{R}} - {\text{C}}( = {\text{O}}) - {\text{R'}}} \right)$.
Thus, compound A must have ${\text{R}} - {\text{C}}( = {\text{O}}) - {\text{C}}{{\text{H}}_3}$ group.
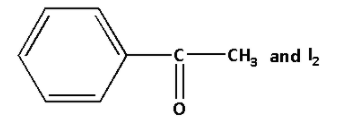
From the given options, option (A) has ${\text{R}} - {\text{C}}( = {\text{O}}) - {\text{C}}{{\text{H}}_3}$ group. The reaction is as follows:
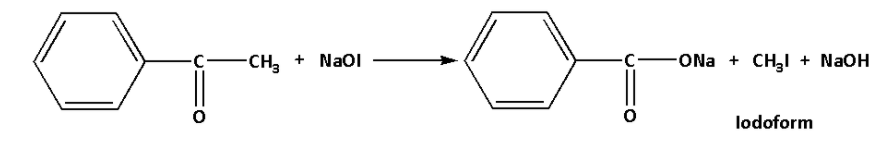
Thus, the above reaction is the reaction of acetophenone sodium hypoiodite to form a yellow coloured precipitate of iodoform. Also sodium benzoate and sodium hydroxide is produced in the reaction.
Thus, the correct option is (A).
Note:
The iodoform test is used as the distinguishing test for methyl ketones. The yellow precipitate formed is the precipitate of iodoform or methyl iodide. Ketones having methyl groups on one side of the carbon-oxygen double bond give a positive iodoform test. The methyl iodide formed in the reaction has a characteristic smell like that of an antiseptic. This test is also known as the haloform test.
Complete step by step solution:
First we will determine the compound Y as follows:
We are given that sodium hypoiodite $\left( {{\text{NaOI}}} \right)$ is produced by the reaction of Y with sodium hydroxide $\left( {{\text{NaOH}}} \right)$. ${\text{NaOI}}$ is produced when ${\text{NaOH}}$ reacts with ${{\text{I}}_2}$. The reaction is as follows:
${\text{NaOH}} + {{\text{I}}_2} \to {\text{NaOI}} + {\text{HI}}$
Thus, compound Y is ${{\text{I}}_2}$.
We are given that compound A $\left( {{{\text{C}}_{\text{8}}}{{\text{H}}_{{\text{10}}}}{\text{O}}} \right)$ reacts with ${\text{NaOI}}$ produces a yellow precipitate.
The yellow precipitate formed in the reaction is of iodoform. The molecular formula of iodoform is ${\text{C}}{{\text{H}}_{\text{3}}}{\text{I}}$.
We know that the iodoform test is positive for methyl ketones. Thus, the compound A must have methyl $\left( { - {\text{C}}{{\text{H}}_3}} \right)$ group and a ketonic group $\left( {{\text{R}} - {\text{C}}( = {\text{O}}) - {\text{R'}}} \right)$.
Thus, compound A must have ${\text{R}} - {\text{C}}( = {\text{O}}) - {\text{C}}{{\text{H}}_3}$ group.
From the given options, option (A) has ${\text{R}} - {\text{C}}( = {\text{O}}) - {\text{C}}{{\text{H}}_3}$ group. The reaction is as follows:

Thus, the above reaction is the reaction of acetophenone sodium hypoiodite to form a yellow coloured precipitate of iodoform. Also sodium benzoate and sodium hydroxide is produced in the reaction.
Thus, the correct option is (A).
Note:
The iodoform test is used as the distinguishing test for methyl ketones. The yellow precipitate formed is the precipitate of iodoform or methyl iodide. Ketones having methyl groups on one side of the carbon-oxygen double bond give a positive iodoform test. The methyl iodide formed in the reaction has a characteristic smell like that of an antiseptic. This test is also known as the haloform test.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




