
Compound A of molecular formula ${{C}_{3}}{{H}_{6}}O$ does not reduce Tollens reagent and Fehling’s solution. Compound A undergoes Clemmensen reduction to give compound B of the molecular formula ${{C}_{3}}{{H}_{8}}$ . Compound A in the presence of conc. ${{H}_{2}}S{{O}_{4}}$ condenses giving aromatic compound C of the molecular formula ${{C}_{9}}{{H}_{12}}$ . Identify B and C. Explain the reactions.
Answer
591.9k+ views
Hint: Clemmensen reduction is a chemical reaction for the reduction of ketones or aldehydes to alkanes using zinc amalgam and concentrated HCl. Fehling’s solution besides Tollen’s reagent is used to differentiate between reducing and non reducing sugars.
Complete answer:
- Tollen’s reagent is a chemical reagent used to identify the presence of aldehyde, aromatic aldehyde, and alpha-hydroxy ketone functional groups.
- Fehling is a deep blue alkaline solution which is used to determine the presence of aldehyde or ketone groups.
- Clemenson reductions are effective at reducing aryl-alkyl ketones.
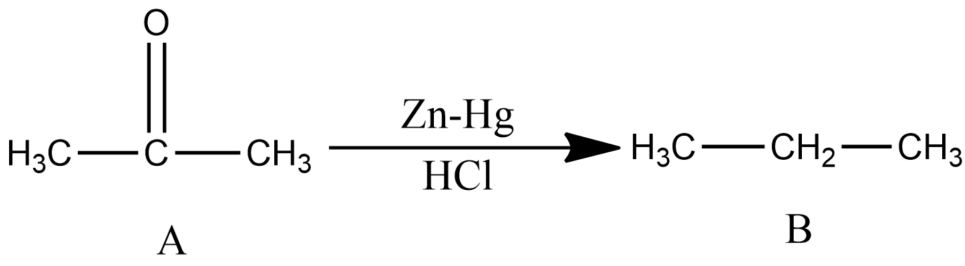
-Since the compound, A with molecular formula ${{C}_{3}}{{H}_{6}}O$ is not reduced by Tollen’s reagent and Fehling’s solution and is undergoing Clemmensen reduction, so it must be ketone (acetone).
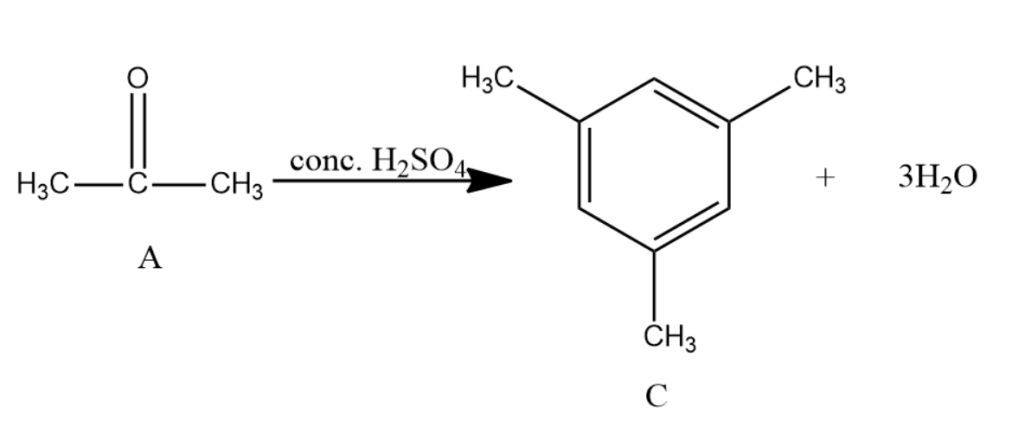
-Acetone is further reacted with conc. ${{H}_{2}}S{{O}_{4}}$ with and distilled water gives Mesitylene which is the compound C. It has the molecular formula as ${{C}_{9}}{{H}_{12}}$ and is an aromatic compound.
Note: You may get confused about compound A thinking it as aldehyde or ketone because the Clemmensen reaction is the reduction reaction for both aldehyde and ketone. Since compound A is not giving any reaction with Tollen’s reagent and Fehling’s solution, this cancels out the chances of compound A being an aldehyde.
Complete answer:
- Tollen’s reagent is a chemical reagent used to identify the presence of aldehyde, aromatic aldehyde, and alpha-hydroxy ketone functional groups.
- Fehling is a deep blue alkaline solution which is used to determine the presence of aldehyde or ketone groups.
- Clemenson reductions are effective at reducing aryl-alkyl ketones.
-Since the compound, A with molecular formula ${{C}_{3}}{{H}_{6}}O$ is not reduced by Tollen’s reagent and Fehling’s solution and is undergoing Clemmensen reduction, so it must be ketone (acetone).
-Acetone is further reacted with conc. ${{H}_{2}}S{{O}_{4}}$ with and distilled water gives Mesitylene which is the compound C. It has the molecular formula as ${{C}_{9}}{{H}_{12}}$ and is an aromatic compound.


Note: You may get confused about compound A thinking it as aldehyde or ketone because the Clemmensen reaction is the reduction reaction for both aldehyde and ketone. Since compound A is not giving any reaction with Tollen’s reagent and Fehling’s solution, this cancels out the chances of compound A being an aldehyde.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




