
Consider the energy profile, for the reaction \[X + Y \to R + S\] which of the following deductions about reaction is not correct?
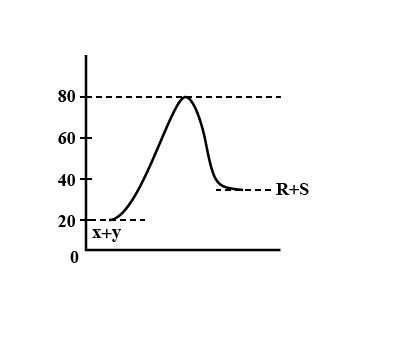
A.The energy of activation for the backward reaction is 80 KJ
B.The forward reaction is Endothermic
C. \[\Delta H\] for the forward reaction is 20 KJ
D.The energy of activation for forward reaction is 60 KJ

Answer
567.9k+ views
Hint: In the case of an endothermic reaction, the activation energy of the reverse reaction will always be smaller than the activation energy of the reverse reaction and it will be opposite for the exothermic reaction.
Complete step by step answer:
For the reaction \[X + Y \to R + S\]
The activation energy for the forward reaction, \[{E^f}_a\] and the activation energy for the backward reaction, Era are related to the enthalpy \[\left( {\Delta H} \right)\] of the reaction by the equation,
\[\Delta H = {E^f}_a - {E^r}_a\]
Energy of activation for forward reaction, \[{E^f}_a = 80-20 = 60KJ\]
Energy of activation for the backward reaction, \[{E^r}_a = 80-40 = 40KJ\]
\[\Delta H = {E^f}_a - {E^r}_a\]
So \[\Delta H = 60-40 = 20KJ\]
In the case of an endothermic reaction, the activation energy of the reverse reaction will always be smaller than the activation energy of the reverse reaction.
i.e. for the endothermic reactions, \[\Delta H > 0\], so that \[{E^r}_a < {E^f}_a\]
In the case of an exothermic reaction, the activation energy of the forward reaction will always be smaller than the activation energy of the reverse reaction.
i.e. for the exothermic reactions, \[\Delta H < 0\], so that \[{E^r}_a > {E^f}_a\]
Hence, all the options except the first one is correct.
Therefore, the correct answer is option (A).
Note: In the case of the reverse reaction, we will have to supply energy to the more reactant in order to get it to reform the less stable, i.e. higher in energy product.
The difference between the activation energy of the forward reaction and the activation energy of the reverse reaction will be \[\Delta H\]. So, for exothermic reactions, we'll always have a bigger \[{E^r}_a\] for the reverse reaction than we had for the forward reaction
Complete step by step answer:
For the reaction \[X + Y \to R + S\]
The activation energy for the forward reaction, \[{E^f}_a\] and the activation energy for the backward reaction, Era are related to the enthalpy \[\left( {\Delta H} \right)\] of the reaction by the equation,
\[\Delta H = {E^f}_a - {E^r}_a\]
Energy of activation for forward reaction, \[{E^f}_a = 80-20 = 60KJ\]
Energy of activation for the backward reaction, \[{E^r}_a = 80-40 = 40KJ\]
\[\Delta H = {E^f}_a - {E^r}_a\]
So \[\Delta H = 60-40 = 20KJ\]
In the case of an endothermic reaction, the activation energy of the reverse reaction will always be smaller than the activation energy of the reverse reaction.
i.e. for the endothermic reactions, \[\Delta H > 0\], so that \[{E^r}_a < {E^f}_a\]
In the case of an exothermic reaction, the activation energy of the forward reaction will always be smaller than the activation energy of the reverse reaction.
i.e. for the exothermic reactions, \[\Delta H < 0\], so that \[{E^r}_a > {E^f}_a\]
Hence, all the options except the first one is correct.
Therefore, the correct answer is option (A).
Note: In the case of the reverse reaction, we will have to supply energy to the more reactant in order to get it to reform the less stable, i.e. higher in energy product.
The difference between the activation energy of the forward reaction and the activation energy of the reverse reaction will be \[\Delta H\]. So, for exothermic reactions, we'll always have a bigger \[{E^r}_a\] for the reverse reaction than we had for the forward reaction
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




