
Consider the following arrangements of the octahedral complex ion ${\left[ {{\text{Co}}{{\left( {{\text{N}}{{\text{H}}_{\text{3}}}} \right)}_{\text{4}}}{\text{C}}{{\text{l}}_{\text{2}}}} \right]^ + }$. Which of the following statements is false?
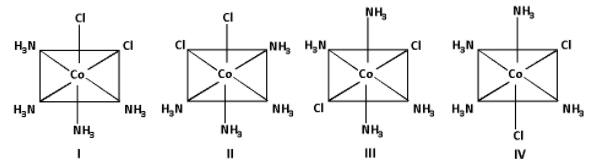
A) II and III are cis and trans-isomers respectively.
B) III and IV are trans and cis-isomers respectively.
C) I and II are enantiomers.
D) All are identical isomers.

Answer
578.4k+ views
Hint: The complex ${\left[ {{\text{Co}}{{\left( {{\text{N}}{{\text{H}}_{\text{3}}}} \right)}_{\text{4}}}{\text{C}}{{\text{l}}_{\text{2}}}} \right]^ + }$ is a ${\left[ {{\text{M}}{{\text{a}}_4}{{\text{b}}_2}} \right]^ + }$ type of complex. In this complex, cis isomers is obtained when the b-type ligands are adjacent to each other and the trans isomer is obtained when the b-type ligands are opposite to each other.
Complete answer:
The complex ${\left[ {{\text{Co}}{{\left( {{\text{N}}{{\text{H}}_{\text{3}}}} \right)}_{\text{4}}}{\text{C}}{{\text{l}}_{\text{2}}}} \right]^ + }$ is a ${\left[ {{\text{M}}{{\text{a}}_4}{{\text{b}}_2}} \right]^ + }$ type of complex. In this complex, the a-type ligand is ${\text{N}}{{\text{H}}_{\text{3}}}$ and the b-type ligand is ${\text{Cl}}$.
The cis isomers is obtained when the b-type ligands are adjacent to each other and the trans isomer is obtained when the b-type ligands are opposite to each other.
In structure II, the b-type ligands i.e. ${\text{Cl}}$ ligands are adjacent to each other. Thus, structure II is a cis isomer.
In structure III, the b-type ligands i.e. ${\text{Cl}}$ ligands are opposite to each other. Thus, structure III is a trans isomer.
Thus, the statement ‘II and III are cis and trans-isomers respectively’ is true. Thus, option (A) is not correct.
In structure III, the b-type ligands i.e. ${\text{Cl}}$ ligands are opposite to each other. Thus, structure III is a trans isomer.
In structure IV, the b-type ligands i.e. ${\text{Cl}}$ ligands are not opposite to each other. Thus, structure IV is not a cis isomer.
Thus, the statement ‘III and IV are trans and cis-isomers respectively’ is false.
The isomers that are non-super imposable mirror images of each other are known as enantiomers.
Thus, the statement ‘structure I and structure II are the enantiomers’ is true. Thus, option (C) is not correct.
The structures having the same molecular formula are said to be identical.
All the structures have the same molecular formula i.e. ${\left[ {{\text{Co}}{{\left( {{\text{N}}{{\text{H}}_{\text{3}}}} \right)}_{\text{4}}}{\text{C}}{{\text{l}}_{\text{2}}}} \right]^ + }$.
Thus, the statement ‘all are identical isomers’ is true. Thus, option (D) is not correct.
Thus, the false statement is ‘III and IV are trans and cis-isomers respectively’.
Thus, the correct option is (B) III and IV are trans and cis-isomers respectively.
Note: The non-super imposable mirror images means that the structures are mirror images of each other but when they are placed over each other they do not superimpose. Such structures are known as enantiomers of each other.
Complete answer:
The complex ${\left[ {{\text{Co}}{{\left( {{\text{N}}{{\text{H}}_{\text{3}}}} \right)}_{\text{4}}}{\text{C}}{{\text{l}}_{\text{2}}}} \right]^ + }$ is a ${\left[ {{\text{M}}{{\text{a}}_4}{{\text{b}}_2}} \right]^ + }$ type of complex. In this complex, the a-type ligand is ${\text{N}}{{\text{H}}_{\text{3}}}$ and the b-type ligand is ${\text{Cl}}$.
The cis isomers is obtained when the b-type ligands are adjacent to each other and the trans isomer is obtained when the b-type ligands are opposite to each other.
In structure II, the b-type ligands i.e. ${\text{Cl}}$ ligands are adjacent to each other. Thus, structure II is a cis isomer.
In structure III, the b-type ligands i.e. ${\text{Cl}}$ ligands are opposite to each other. Thus, structure III is a trans isomer.
Thus, the statement ‘II and III are cis and trans-isomers respectively’ is true. Thus, option (A) is not correct.
In structure III, the b-type ligands i.e. ${\text{Cl}}$ ligands are opposite to each other. Thus, structure III is a trans isomer.
In structure IV, the b-type ligands i.e. ${\text{Cl}}$ ligands are not opposite to each other. Thus, structure IV is not a cis isomer.
Thus, the statement ‘III and IV are trans and cis-isomers respectively’ is false.
The isomers that are non-super imposable mirror images of each other are known as enantiomers.
Thus, the statement ‘structure I and structure II are the enantiomers’ is true. Thus, option (C) is not correct.
The structures having the same molecular formula are said to be identical.
All the structures have the same molecular formula i.e. ${\left[ {{\text{Co}}{{\left( {{\text{N}}{{\text{H}}_{\text{3}}}} \right)}_{\text{4}}}{\text{C}}{{\text{l}}_{\text{2}}}} \right]^ + }$.
Thus, the statement ‘all are identical isomers’ is true. Thus, option (D) is not correct.
Thus, the false statement is ‘III and IV are trans and cis-isomers respectively’.
Thus, the correct option is (B) III and IV are trans and cis-isomers respectively.
Note: The non-super imposable mirror images means that the structures are mirror images of each other but when they are placed over each other they do not superimpose. Such structures are known as enantiomers of each other.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

What is a transformer Explain the principle construction class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE




