
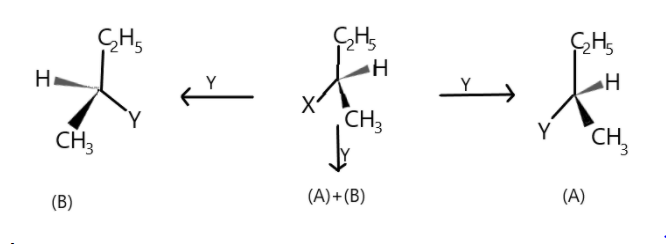
Consider the three types of replacement of group $X$ by group $Y$ as shown here. This can result in giving compound (A) or (B) or both. What is the process called if (B) is the only compound obtained.

Answer
573.3k+ views
Hint: As we know that the configuration of compounds change when a nucleophile attacks the carbon atoms following the substitution nucleophile $S{N^1}$ reaction because a nucleophile can attack from any face and rare side of the carbocation as the substituent is planar to the carbocation. The relative configuration of the compound is retained.
Complete step by step solution: The process is called Retention. And as we know that retention is the process in which the relative configuration is retained. The reaction involves the replacement of a ligand on a chiral center in a reactant molecule and if the product occupies the same site on the chiral center as the replaced ligand did in the reactant, it is said to be the retention of configuration.
As we know that racemisation is conversion of optically active compounds into a mixture of enantiomers with no optical activity and in $S{N^1}$reaction the rate limiting step is the formation of carbocation and second step involves the attack of the nucleophile on the carbocation. So this nucleophile can attack from any face of the carbocation because the carbocation and substituents are planar but when it attacks from the rare side there is inversion of configuration of compound which results in the formation of one type of isomer and when nucleophile attacks from front there is retention of configuration leading to the formation of mirror image of the isomer. So the two isomers formed are in equal concentration as the probability of attack is $1:1$ but have opposite optical activity cancelling out one another’s optical activity and make the compound optically inactive. This mixture is thus called racemic mixture as in $S{N^1}$ the nucleophile has the ability to attack from either side.
Thus the process is called Retention or racemisation.
Note: Although racemisation is involved in $S{N^1}$ but it is not $100\% $ as the carbocation formed is not stable so it is possible that nucleophiles can attack from the back side of the carbocation and the front side may be shielded by the leaving group.
Complete step by step solution: The process is called Retention. And as we know that retention is the process in which the relative configuration is retained. The reaction involves the replacement of a ligand on a chiral center in a reactant molecule and if the product occupies the same site on the chiral center as the replaced ligand did in the reactant, it is said to be the retention of configuration.
As we know that racemisation is conversion of optically active compounds into a mixture of enantiomers with no optical activity and in $S{N^1}$reaction the rate limiting step is the formation of carbocation and second step involves the attack of the nucleophile on the carbocation. So this nucleophile can attack from any face of the carbocation because the carbocation and substituents are planar but when it attacks from the rare side there is inversion of configuration of compound which results in the formation of one type of isomer and when nucleophile attacks from front there is retention of configuration leading to the formation of mirror image of the isomer. So the two isomers formed are in equal concentration as the probability of attack is $1:1$ but have opposite optical activity cancelling out one another’s optical activity and make the compound optically inactive. This mixture is thus called racemic mixture as in $S{N^1}$ the nucleophile has the ability to attack from either side.
Thus the process is called Retention or racemisation.
Note: Although racemisation is involved in $S{N^1}$ but it is not $100\% $ as the carbocation formed is not stable so it is possible that nucleophiles can attack from the back side of the carbocation and the front side may be shielded by the leaving group.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




