
Construct the formula of the following compounds using criss crossing valency.
(a) Calcium hydroxide
(b) Ammonium carbonate
(c) Zinc oxide
(d) Aluminium oxide
Answer
531.9k+ views
Hint: The Criss Cross valency method is used to write the chemical formula of ionic compounds. In this method, the numeric value of charges present on each element is crossed over and put as the subscript of another ion.
Complete answer:
Ionic compounds are made up of a cationic and an anionic part but the overall compound remains electrically neutral. The Criss Cross valency method is one of the ways to write formulas of such compounds that is discussed below.
(1) - To write the formula of ionic compounds, we start by writing the symbol of cation and anion separately. The action should be written first.
(2) - Then, put only the numeric values of ionic charge of each just below its symbol.
(3) - Then, transpose the charge of cation as a subscript to the anion and the charge of anion will become the subscript of the cation.
(4) - Reduce the formula to its simplest ratio.
(5) - Write the formula of the compound and simply drop the subscript ‘1’, if present.
(6) - If polyatomic ions are present, then put them in curved brackets.
Now, we know how to use the Crisscross method to write the formula of compounds. Let us write the formula of the given compounds by following these steps.
(a) Calcium hydroxide
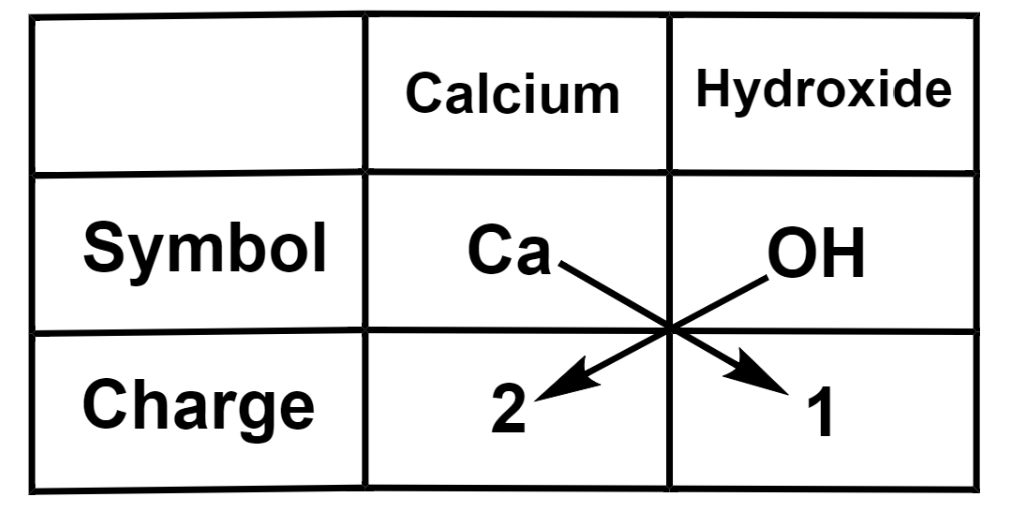
Therefore, we get the formula of calcium hydroxide as $\text{Ca}{{\left( \text{OH} \right)}_{2}}$.
(b) Ammonium carbonate
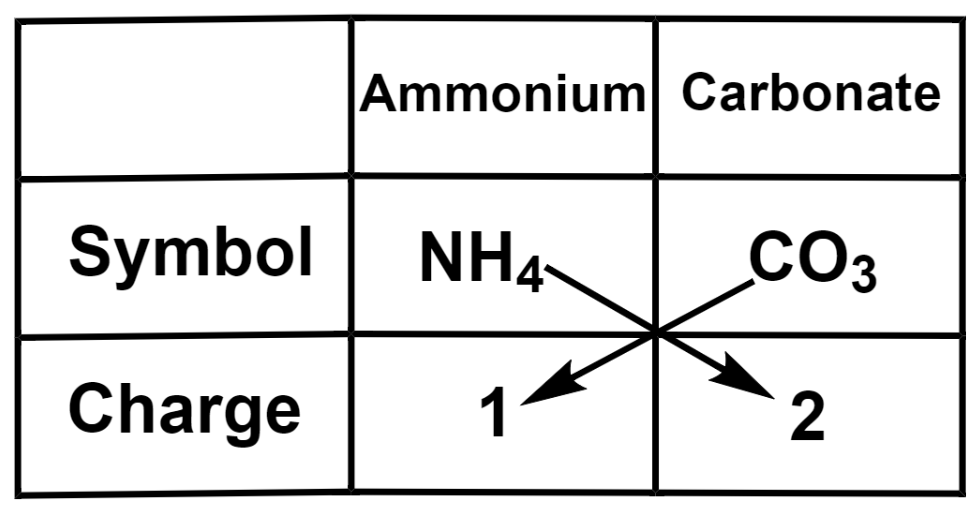
Therefore, we get the formula of ammonium carbonate as ${{\left( \text{N}{{\text{H}}_{4}} \right)}_{2}}\text{C}{{\text{O}}_{3}}$.
(c) Zinc oxide
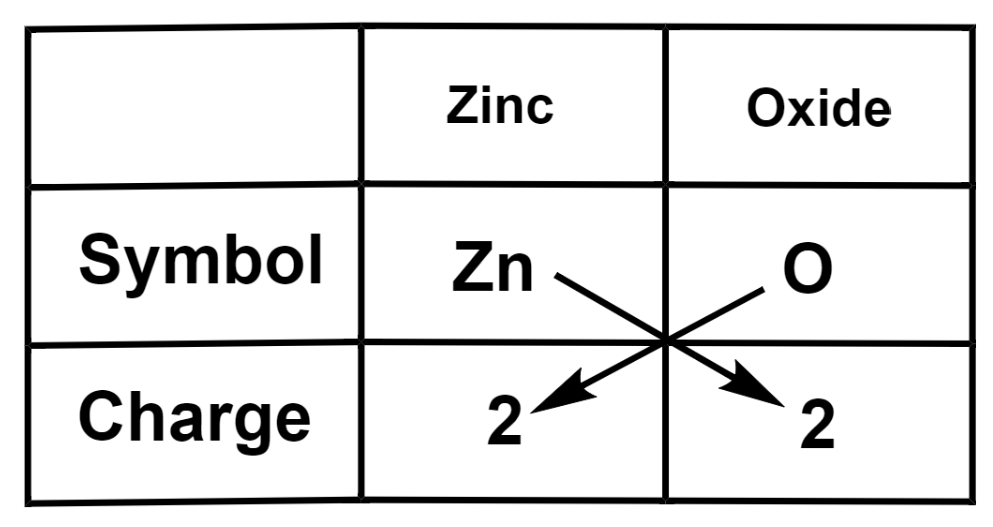
We get the formula of zinc oxide as $\text{Z}{{\text{n}}_{2}}{{\text{O}}_{2}}$. After reducing to the simplest ratio, the actual formula of zinc oxide is ZnO.
(d) Aluminium oxide
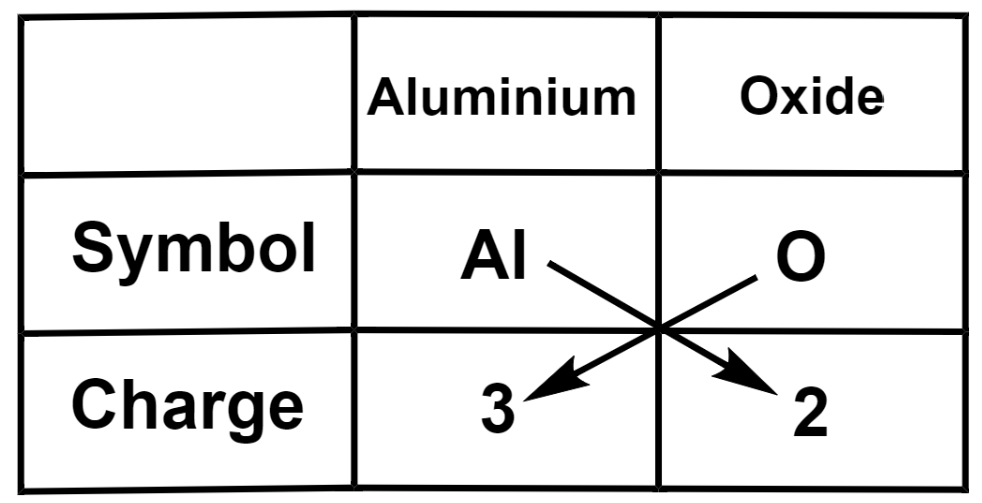
Therefore, we get the formula of aluminium oxide as $\text{A}{{\text{l}}_{2}}{{\text{O}}_{3}}$.
Note:
The ionic compounds exist as a crystal lattice consisting of many ions of cation and anion. The formula of these compounds is thus an empirical formula which only indicates what ratio of anions and cations are present in a compound.
Complete answer:
Ionic compounds are made up of a cationic and an anionic part but the overall compound remains electrically neutral. The Criss Cross valency method is one of the ways to write formulas of such compounds that is discussed below.
(1) - To write the formula of ionic compounds, we start by writing the symbol of cation and anion separately. The action should be written first.
(2) - Then, put only the numeric values of ionic charge of each just below its symbol.
(3) - Then, transpose the charge of cation as a subscript to the anion and the charge of anion will become the subscript of the cation.
(4) - Reduce the formula to its simplest ratio.
(5) - Write the formula of the compound and simply drop the subscript ‘1’, if present.
(6) - If polyatomic ions are present, then put them in curved brackets.
Now, we know how to use the Crisscross method to write the formula of compounds. Let us write the formula of the given compounds by following these steps.
(a) Calcium hydroxide

Therefore, we get the formula of calcium hydroxide as $\text{Ca}{{\left( \text{OH} \right)}_{2}}$.
(b) Ammonium carbonate

Therefore, we get the formula of ammonium carbonate as ${{\left( \text{N}{{\text{H}}_{4}} \right)}_{2}}\text{C}{{\text{O}}_{3}}$.
(c) Zinc oxide

We get the formula of zinc oxide as $\text{Z}{{\text{n}}_{2}}{{\text{O}}_{2}}$. After reducing to the simplest ratio, the actual formula of zinc oxide is ZnO.
(d) Aluminium oxide

Therefore, we get the formula of aluminium oxide as $\text{A}{{\text{l}}_{2}}{{\text{O}}_{3}}$.
Note:
The ionic compounds exist as a crystal lattice consisting of many ions of cation and anion. The formula of these compounds is thus an empirical formula which only indicates what ratio of anions and cations are present in a compound.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

10 examples of friction in our daily life




