
Conversion of phenol to salicylic acid and to salicyaldehyde are known as? (respectively)
(A) Reimer-Tiemann reaction and Kolbe’s reaction
(B) Williamson’s synthesis and Hydroboration-oxidation
(C) Kolbe’s reaction and Williamson’s synthesis
(D) Kolbe’s reaction and Reimer-Tiemann reaction
Answer
530.8k+ views
Hint:The conversion of phenol to salicylic acid takes place through a base, and hydrolysis. If we talk about the conversion of phenol of salicylaldehyde takes place through the action of chloroform. So, we identify the naming reactions in which these conversions take place.
Complete step by step solution:
First, we will discuss the conversion of phenol to salicylic acid.
The phenol will be deprotonated by strong base i.e. hydroxide ion. There will be addition of phenoxide ions formed to the carbon-dioxide.
So, we can say that the addition further leads to the carboxylate formation. Then, it will further lead to the formation of carboxylic acid, i.e. salicylic acid.
The chemical reaction is
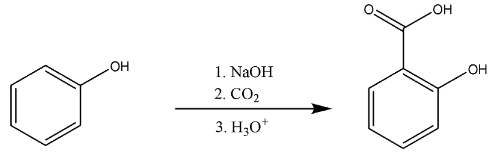
Thus, we know this whole mechanism takes place in Kolbe’s reaction, it is used for the synthesis of aspirin also known as salicylic acid in the industries.
Now, the next we have conversion of phenol to salicylaldehyde. It was proposed by Karl Riemer, mainly used for the ortho- phenols.
In this reaction too like Kolbe’s reaction, there is deprotonation of chloroform by strong base like KOH.
This reaction takes place through the nucleophilic attack, and the basic hydrolysis is done, leading to the formation of salicylaldehyde.
The chemical reaction is
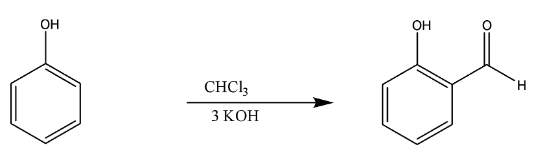
Thus, we can say this whole mechanism takes place in the Riemer –Tiemann reaction.
In the last, we can conclude that conversion of phenol to salicylic acid and to salicylaldehyde are known as Kolbe’ reaction, and Riemer-Tiemann reaction.
Hence, the correct option is (D).
Note: Don’t get confused between both these reactions. These both takes place through deprotonation by a strong base, as we discussed; but the product formation will be different. In case 1 there is addition of carbon-dioxide, and in case 2 there is nucleophilic attack.
Complete step by step solution:
First, we will discuss the conversion of phenol to salicylic acid.
The phenol will be deprotonated by strong base i.e. hydroxide ion. There will be addition of phenoxide ions formed to the carbon-dioxide.
So, we can say that the addition further leads to the carboxylate formation. Then, it will further lead to the formation of carboxylic acid, i.e. salicylic acid.
The chemical reaction is

Thus, we know this whole mechanism takes place in Kolbe’s reaction, it is used for the synthesis of aspirin also known as salicylic acid in the industries.
Now, the next we have conversion of phenol to salicylaldehyde. It was proposed by Karl Riemer, mainly used for the ortho- phenols.
In this reaction too like Kolbe’s reaction, there is deprotonation of chloroform by strong base like KOH.
This reaction takes place through the nucleophilic attack, and the basic hydrolysis is done, leading to the formation of salicylaldehyde.
The chemical reaction is

Thus, we can say this whole mechanism takes place in the Riemer –Tiemann reaction.
In the last, we can conclude that conversion of phenol to salicylic acid and to salicylaldehyde are known as Kolbe’ reaction, and Riemer-Tiemann reaction.
Hence, the correct option is (D).
Note: Don’t get confused between both these reactions. These both takes place through deprotonation by a strong base, as we discussed; but the product formation will be different. In case 1 there is addition of carbon-dioxide, and in case 2 there is nucleophilic attack.
Recently Updated Pages
JEE Main 2023 April 6 Shift 1 Question Paper with Answer Key

JEE Main 2023 April 6 Shift 2 Question Paper with Answer Key

JEE Main 2023 (January 31 Evening Shift) Question Paper with Solutions [PDF]

JEE Main 2023 January 30 Shift 2 Question Paper with Answer Key

JEE Main 2023 January 25 Shift 1 Question Paper with Answer Key

JEE Main 2023 January 24 Shift 2 Question Paper with Answer Key

Trending doubts
JEE Main 2026: Session 2 Registration Open, City Intimation Slip, Exam Dates, Syllabus & Eligibility

JEE Main 2026 Application Login: Direct Link, Registration, Form Fill, and Steps

Understanding the Angle of Deviation in a Prism

Hybridisation in Chemistry – Concept, Types & Applications

How to Convert a Galvanometer into an Ammeter or Voltmeter

Understanding the Electric Field of a Uniformly Charged Ring

Other Pages
JEE Advanced Marks vs Ranks 2025: Understanding Category-wise Qualifying Marks and Previous Year Cut-offs

NCERT Solutions For Class 12 Chemistry Chapter 1 Solutions (2025-26)

Solutions Class 12 Chemistry Chapter 1 CBSE Notes - 2025-26

NCERT Solutions For Class 12 Chemistry Chapter 4 The d and f Block Elements (2025-26)

Biomolecules Class 12 Chemistry Chapter 10 CBSE Notes - 2025-26

NCERT Solutions For Class 12 Chemistry Chapter 10 Biomolecules (2025-26)




