
How do you convert:
1.Phenol to toluene.
2.Formaldehyde to Ethanol.
Answer
513k+ views
Hint: We know that there are many paths by which phenol can be converted into toluene It may involve first substitution of methyl group on benzene ring and then its oxidation and then further conversion with use of reagents. Similarly, toluene can be oxidised first and then converted into phenol.
Complete answer:
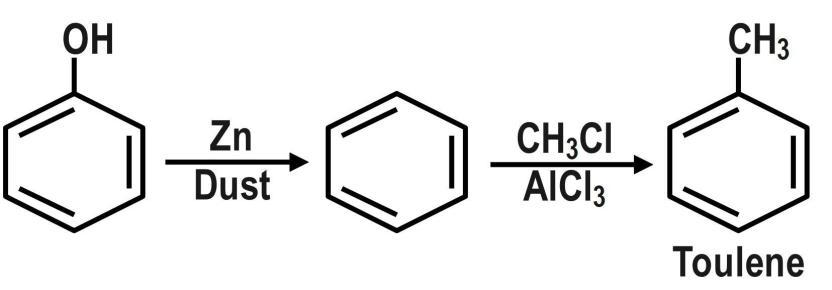
Benzene is an electron rich compound. It is resonance stabilized, so it cannot undergo addition reactions because addition will lead to destroy of resonance. But it undergoes electrophilic substitution reactions where one Hydrogen is substituted by any other group. The first step involves the Friedel Crafts alkylation reaction in which methyl chloride and is used to convert phenol into toluene. Then, the Toluene is converted to benzoic acid by acidifying Potassium dichromate. The reaction is as:
We can convert methanol into ethanol by an organic coupling reaction which is called Wurtz reaction. This is carried out with sodium metal in the presence of dry ether and forms higher alkanes. Wurtz reaction is the classic coupling reaction in which halo-alkanes are created to higher alkanes in presence of sodium atoms and dry ether. Single electron transfer takes place from sodium metal to the alkyl halide dissociating to form the alkyl radical and sodium halide salt. It is one of the oldest coupling reactions.
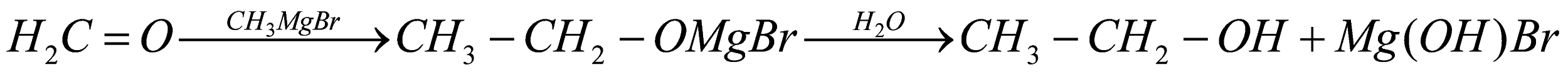
Note:
Remember that the conversion of toluene to benzaldehyde by use of reagent is termed as Etard reaction. This reaction is used to produce benzaldehyde for many purposes. The reaction of benzaldehyde with hydrogen peroxide and sodium hydroxide is named as Dakin reaction. It is commonly used to prepare phenol. Another method to convert methanol into ethanol is by using Grignard reagent. We can first add Grignard Reagent with PCC (Pyridinium Chlorochromate, also called Corey’s reagent) to give (Formaldehyde). The Grignard reagent further undergoes acid hydrolysis and reacts with HCHO formed results in ethanol.
Complete answer:
Benzene is an electron rich compound. It is resonance stabilized, so it cannot undergo addition reactions because addition will lead to destroy of resonance. But it undergoes electrophilic substitution reactions where one Hydrogen is substituted by any other group. The first step involves the Friedel Crafts alkylation reaction in which methyl chloride and is used to convert phenol into toluene. Then, the Toluene is converted to benzoic acid by acidifying Potassium dichromate. The reaction is as:

We can convert methanol into ethanol by an organic coupling reaction which is called Wurtz reaction. This is carried out with sodium metal in the presence of dry ether and forms higher alkanes. Wurtz reaction is the classic coupling reaction in which halo-alkanes are created to higher alkanes in presence of sodium atoms and dry ether. Single electron transfer takes place from sodium metal to the alkyl halide dissociating to form the alkyl radical and sodium halide salt. It is one of the oldest coupling reactions.
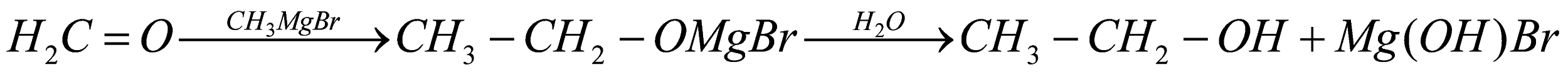
Note:
Remember that the conversion of toluene to benzaldehyde by use of reagent is termed as Etard reaction. This reaction is used to produce benzaldehyde for many purposes. The reaction of benzaldehyde with hydrogen peroxide and sodium hydroxide is named as Dakin reaction. It is commonly used to prepare phenol. Another method to convert methanol into ethanol is by using Grignard reagent. We can first add Grignard Reagent with PCC (Pyridinium Chlorochromate, also called Corey’s reagent) to give (Formaldehyde). The Grignard reagent further undergoes acid hydrolysis and reacts with HCHO formed results in ethanol.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




