
How will you convert-
(a) Ethanoic acid into propanoic acid
(b) Propanoic acid into ethanoic acid
Answer
598.2k+ views
Hint: We know that for the conversion of the given compounds we need to perform several reactions in order to get our desired products. While performing these chain reactions we need to remember some important reactions where we need to reduce or increase the number of carbon atoms.
Complete step by step solution:
(a) Ethanoic acid into propanoic acid – We know that carboxylic acids can be converted into alcohols by treating them with lithium aluminium hydride, so here we have treated ethanoic acid with $LiAl{H_4}$ and got ethyl alcohol. When ethyl alcohol is treated with potassium iodide it produces ethyl iodide which further takes part in the reaction with potassium cyanide and produces ethyl cyanide. At last when ethyl cyanide reacts with ${H_2}O/{H^ + }$ it gives propanoic acid.
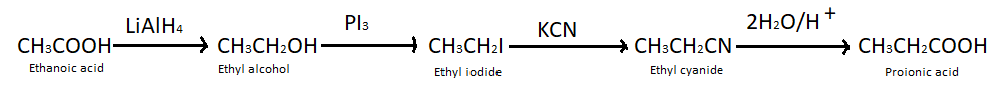
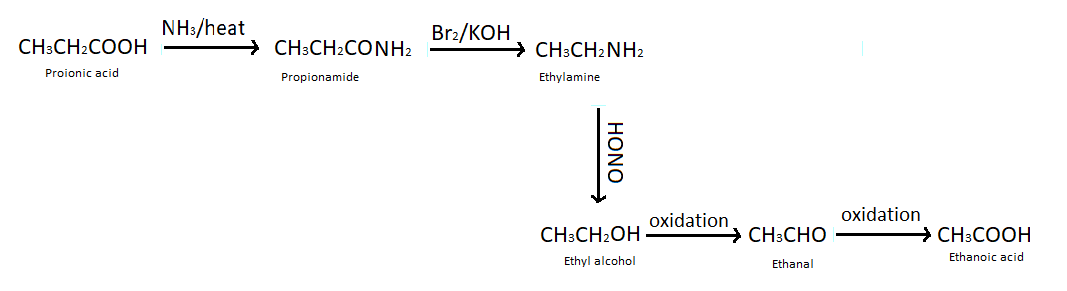
(b) Propanoic acid into ethanoic acid - To convert propanoic acid into ethanoic acid first of all we heat propanoic acid with ammonia which produces propenamide (an ammonium salt). Then we treat propenamide with bromine and aqueous or alcoholic $KOH$ which gives ethylamine, so here one carbon atom is reduced from the compound. This reaction is also known as Hoffmann bromamide degradation reaction. We have studied that when nitrous acid reacts with amine it produces alcohol so we have treated ethyl amine with nitrous acid. After all of these reactions by performing oxidation of ethyl alcohol we got ethanol and again doing oxidation reaction we got our desired product that is ethanoic acid.
Note : Hence we have converted ethanoic acid into propanoic acids and propanoic acid into ethanoic acid with the help of reactions which is mentioned above in the solution.
Complete step by step solution:
(a) Ethanoic acid into propanoic acid – We know that carboxylic acids can be converted into alcohols by treating them with lithium aluminium hydride, so here we have treated ethanoic acid with $LiAl{H_4}$ and got ethyl alcohol. When ethyl alcohol is treated with potassium iodide it produces ethyl iodide which further takes part in the reaction with potassium cyanide and produces ethyl cyanide. At last when ethyl cyanide reacts with ${H_2}O/{H^ + }$ it gives propanoic acid.
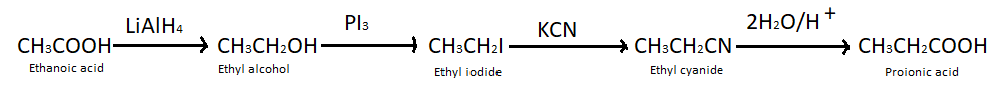
(b) Propanoic acid into ethanoic acid - To convert propanoic acid into ethanoic acid first of all we heat propanoic acid with ammonia which produces propenamide (an ammonium salt). Then we treat propenamide with bromine and aqueous or alcoholic $KOH$ which gives ethylamine, so here one carbon atom is reduced from the compound. This reaction is also known as Hoffmann bromamide degradation reaction. We have studied that when nitrous acid reacts with amine it produces alcohol so we have treated ethyl amine with nitrous acid. After all of these reactions by performing oxidation of ethyl alcohol we got ethanol and again doing oxidation reaction we got our desired product that is ethanoic acid.

Note : Hence we have converted ethanoic acid into propanoic acids and propanoic acid into ethanoic acid with the help of reactions which is mentioned above in the solution.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




