
How to convert Aniline to nitrobenzene?
Answer
547.5k+ views
Hint: Aniline cannot be directly converted to nitrobenzene directly. It needs to be converted to a diazonium salt first which can then be treated with other chemical agents to form the nitrobenzene.
Complete Stepwise Solution:
Aniline is a basic molecule which comprises an amino group attached to the benzene ring. This can be treated with a cold dilute acidic solution sodium nitrite in the temperature range of $ {\left( {{\text{0 - 5}}} \right)^{{\text{ 0}}}}{\text{C}} $ to form diazo compounds which form the intermediate for the transformation reaction. This is then treated with hydrogen fluoroborate or $ {\text{HB}}{{\text{F}}_{\text{4}}} $ to form the fluro salt of the diazo compound. Then this salt is treated with copper and sodium nitrite to form nitrobenzene. The reaction can be written as follows:
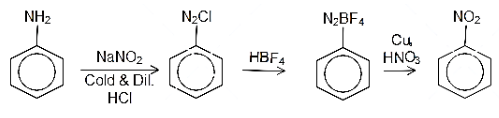
Alternatively, there is one more route for the preparation of nitrobenzene from aniline. aniline can be treated with a cold dilute acidic solution sodium nitrite in the temperature range of $ {\left( {{\text{0 - 5}}} \right)^{{\text{ 0}}}}{\text{C}} $ to form diazo compound. Then diazonium chloride is then hydrolysed in presence of hypophosphorous acid and water to form benzene. The benzene is then treated with concentrated solution of sulphuric acid and nitric acid to form the nitrobenzene. The reaction can be written as follows:
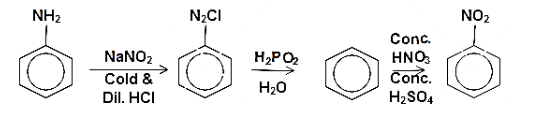
Note:
The reaction of Aniline with the cold dilute acidic solution of sodium nitrate to form the diazonium salt serves to be an intermediate for the preparation of a number of compounds. The diazo salt is not stable at high temperatures and hence the reaction takes place at cold temperature conditions.
Complete Stepwise Solution:
Aniline is a basic molecule which comprises an amino group attached to the benzene ring. This can be treated with a cold dilute acidic solution sodium nitrite in the temperature range of $ {\left( {{\text{0 - 5}}} \right)^{{\text{ 0}}}}{\text{C}} $ to form diazo compounds which form the intermediate for the transformation reaction. This is then treated with hydrogen fluoroborate or $ {\text{HB}}{{\text{F}}_{\text{4}}} $ to form the fluro salt of the diazo compound. Then this salt is treated with copper and sodium nitrite to form nitrobenzene. The reaction can be written as follows:

Alternatively, there is one more route for the preparation of nitrobenzene from aniline. aniline can be treated with a cold dilute acidic solution sodium nitrite in the temperature range of $ {\left( {{\text{0 - 5}}} \right)^{{\text{ 0}}}}{\text{C}} $ to form diazo compound. Then diazonium chloride is then hydrolysed in presence of hypophosphorous acid and water to form benzene. The benzene is then treated with concentrated solution of sulphuric acid and nitric acid to form the nitrobenzene. The reaction can be written as follows:

Note:
The reaction of Aniline with the cold dilute acidic solution of sodium nitrate to form the diazonium salt serves to be an intermediate for the preparation of a number of compounds. The diazo salt is not stable at high temperatures and hence the reaction takes place at cold temperature conditions.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

10 examples of friction in our daily life




