
How do you convert benzaldehyde to benzophenone in exactly two steps?
Answer
547.8k+ views
Hint:As we know that organic compounds can be synthesised with the help of organic reaction. But in this question, it is mentioned that we have to convert these compounds in not more than two steps. Hence there are many reactions in chemistry that would give the desired product.
Complete step-by-step answer: Chemical reactions are defined as the reaction in which reactant bonds are broken and new bonds are formed within product molecules. It results in the formation of new substances.
In this question, we have to convert benzaldehyde into benzophenone in two steps-
Let us see the conversion,
Step One: in this step, the benzaldehyde is reacted with Grignard reagent followed by hydrolysis to get the desired result. Here, benzaldehyde is reacted with phenyl magnesium bromide. After that water is added and results in the formation of diphenylmethanol.
Let us see the reaction,
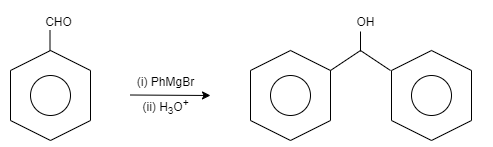
Step Two: in this step, the diphenylmethanol is oxidised by adding the reagent known as chromium trioxide. It results in the formation of benzophenone.
Let us see the reaction,
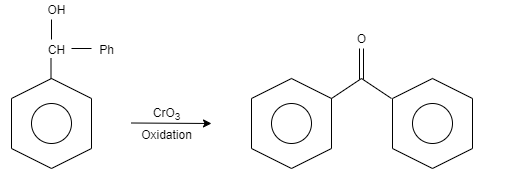
Additional information:
Benzophenone is a white solid organic compound that is soluble in organic solvents. It is widely used building block in organic chemistry.
Benzaldehyde is a colourless liquid that has almonds like odour. It is the simplest aromatic aldehyde.
Note:We should know about Grignard reagent. It is a strong nucleophile that behaves as a carbonyl compound having electrophiles. It can form carbon- carbon bonds. The formula of Grignard reagent is alkyl magnesium halide or aryl magnesium halide.
Complete step-by-step answer: Chemical reactions are defined as the reaction in which reactant bonds are broken and new bonds are formed within product molecules. It results in the formation of new substances.
In this question, we have to convert benzaldehyde into benzophenone in two steps-
Let us see the conversion,
Step One: in this step, the benzaldehyde is reacted with Grignard reagent followed by hydrolysis to get the desired result. Here, benzaldehyde is reacted with phenyl magnesium bromide. After that water is added and results in the formation of diphenylmethanol.
Let us see the reaction,

Step Two: in this step, the diphenylmethanol is oxidised by adding the reagent known as chromium trioxide. It results in the formation of benzophenone.
Let us see the reaction,

Additional information:
Benzophenone is a white solid organic compound that is soluble in organic solvents. It is widely used building block in organic chemistry.
Benzaldehyde is a colourless liquid that has almonds like odour. It is the simplest aromatic aldehyde.
Note:We should know about Grignard reagent. It is a strong nucleophile that behaves as a carbonyl compound having electrophiles. It can form carbon- carbon bonds. The formula of Grignard reagent is alkyl magnesium halide or aryl magnesium halide.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




