
How to convert benzene Diazonium chloride to phenol?
Answer
505.2k+ views
Hint: Benzene Diazonium chloride is an organic compound with the formula $[{C_6}{H_5}{N_2}]Cl$. It is a salt of a Diazonium cation and chloride. It exists as a colorless solid that is soluble in polar solvent including water. Its molar mass is $140.57g/mo{l^ - }$.
Complete answer:
If temperature is increased in aqueous benzene Diazonium chloride, it decomposes to phenol. Therefore, benzene Diazonium is prepared when it is required for some purpose. There are no melting point values because it decomposes readily.
To get phenol from benzene Diazonium chloride, all you need is to warm the benzene Diazonium chloride solution. The Diazonium ion reacts with water in the solution or as a black oily liquid. Nitrogen gas has evolved.
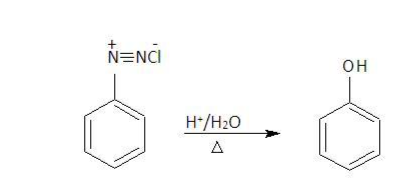
This is the same reaction that you get if you react phenylamine with nitrous acid in warm air. The Diazonium ion is formed first and then immediately reacts with the water in the solution to give phenol.
This compound is prepared by diazotization of aniline in the presence of hydrochloric acid. The conversion involve in situ production of nitrous acid which react with the aniline:
${C_6}{H_5}N{H_2} + HN{O_2} + HCl \to [{C_6}{H_5}{N_2}]Cl + 2{H_2}O$
The reactions are conducted at low temperature to minimize decomposition of the Diazonium salt. The Diazonium salt is not isolated.
The diazo group $({N_2})$ can be replaced by many other groups, usually anions, giving a variety of substituted phenyl derivatives:
${C_6}{H_5}{N_2}^ + + N{u^ - } \to {C_6}{H_5}Nu + {N_2}$
We can categorize reactions of benzenediazonium chloride into two categories:
$1.$ Reaction of substituting Diazonium group by another group
$2.$ Coupling reactions of Diazonium ions.
Benzene Diazonium chloride is used as a raw material in the production of dyes.
Note:
These transformations are associated with many named reactions including the Schiemann reaction, Sandmeyer reaction, and Gomberg-Bachmann reaction. A wide range of groups that can be used to replace ${N_2}$ including halides of considerable practical value in dyes.
Complete answer:

If temperature is increased in aqueous benzene Diazonium chloride, it decomposes to phenol. Therefore, benzene Diazonium is prepared when it is required for some purpose. There are no melting point values because it decomposes readily.
To get phenol from benzene Diazonium chloride, all you need is to warm the benzene Diazonium chloride solution. The Diazonium ion reacts with water in the solution or as a black oily liquid. Nitrogen gas has evolved.

This is the same reaction that you get if you react phenylamine with nitrous acid in warm air. The Diazonium ion is formed first and then immediately reacts with the water in the solution to give phenol.
This compound is prepared by diazotization of aniline in the presence of hydrochloric acid. The conversion involve in situ production of nitrous acid which react with the aniline:
${C_6}{H_5}N{H_2} + HN{O_2} + HCl \to [{C_6}{H_5}{N_2}]Cl + 2{H_2}O$
The reactions are conducted at low temperature to minimize decomposition of the Diazonium salt. The Diazonium salt is not isolated.
The diazo group $({N_2})$ can be replaced by many other groups, usually anions, giving a variety of substituted phenyl derivatives:
${C_6}{H_5}{N_2}^ + + N{u^ - } \to {C_6}{H_5}Nu + {N_2}$
We can categorize reactions of benzenediazonium chloride into two categories:
$1.$ Reaction of substituting Diazonium group by another group
$2.$ Coupling reactions of Diazonium ions.
Benzene Diazonium chloride is used as a raw material in the production of dyes.
Note:
These transformations are associated with many named reactions including the Schiemann reaction, Sandmeyer reaction, and Gomberg-Bachmann reaction. A wide range of groups that can be used to replace ${N_2}$ including halides of considerable practical value in dyes.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




