
How to convert benzene into chlorobenzene?
Answer
507.6k+ views
Hint: Benzene is an organic compound formed by the carbon atoms joint in a plane. Benzene is a colourless or light yellow liquid at room temperature. Benzene has a very sweet odour and is a volatile liquid.
Complete answer:
Benzene being a volatile substance, evaporates as soon as it comes in contact with air. It has a molecular formula of \[{C_6}{H_6}\] .
Now, let’s discuss the structure and the properties of chlorobenzene.
So chlorobenzene is an organic aromatic compound with a molecular formula of \[{C_6}{H_5}Cl\] i.e. one hydrogen atom of the benzene is replaced by the chlorine atom.
And we have to give emphasis on this reaction, so let’s understand how to convert benzene to chlorobenzene.
The reaction to convert benzene to chloro benzene will be:
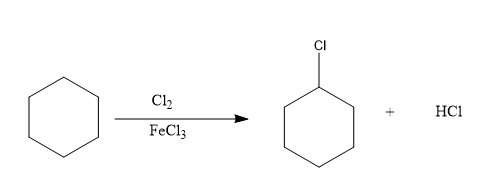
Hence, this is the desired reaction.
Therefore, when Benzene reacts with Chloride in the presence of ferric chloride, the resultant product will be chlorobenzene and hydrochloric acid.
We can also use anhydrous aluminium chloride, \[AlC{l_3}\] in place of ferric chloride as the catalyst.
Hence, we are very much clear with the conversion process or method of chlorobenzene from the benzene. The method is simple and efficient.
Note:
Chloro benzene is known by many different names, so let’s know about all of them to avoid the confusion with these names. Thus, chlorobenzene is also known as mono chlorobenzene , benzene chloride, chloro benzyl and phenyl chloride. Chlorobenzene is basically used as solvents for adhesives and it has paramount importance in the paint industry.
Complete answer:
Benzene being a volatile substance, evaporates as soon as it comes in contact with air. It has a molecular formula of \[{C_6}{H_6}\] .
Now, let’s discuss the structure and the properties of chlorobenzene.
So chlorobenzene is an organic aromatic compound with a molecular formula of \[{C_6}{H_5}Cl\] i.e. one hydrogen atom of the benzene is replaced by the chlorine atom.
And we have to give emphasis on this reaction, so let’s understand how to convert benzene to chlorobenzene.
The reaction to convert benzene to chloro benzene will be:

Hence, this is the desired reaction.
Therefore, when Benzene reacts with Chloride in the presence of ferric chloride, the resultant product will be chlorobenzene and hydrochloric acid.
We can also use anhydrous aluminium chloride, \[AlC{l_3}\] in place of ferric chloride as the catalyst.
Hence, we are very much clear with the conversion process or method of chlorobenzene from the benzene. The method is simple and efficient.
Note:
Chloro benzene is known by many different names, so let’s know about all of them to avoid the confusion with these names. Thus, chlorobenzene is also known as mono chlorobenzene , benzene chloride, chloro benzyl and phenyl chloride. Chlorobenzene is basically used as solvents for adhesives and it has paramount importance in the paint industry.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




