
How do you convert benzoic acid to benzaldehyde?
Answer
520.8k+ views
Hint: The answer lies in the fact that carboxylic group is not directly converted to aldehydic group but it goes via formation of acyl chloride which further undergoes reduction with help of Lindaler’s catalyst.
Complete step by step solution:
In our previous sections of organic chemistry we have studied the reduction reaction which involves a named reaction called Rosenmund's reduction.
- Initially the reaction is followed via conversion of benzoic acid to benzoyl chloride on treatment with phosphorus pentachloride.
- This reagent converts an acidic group to the chloride group which is called a chlorination reaction. Therefore, this reaction is as shown below
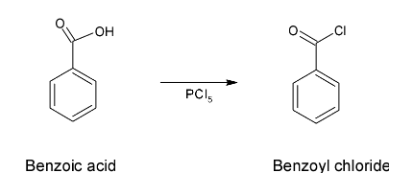
Now, after this conversion, since -Cl group is a better leaving group it easily undergoes a reduction reaction in the presence of Lindlar’s catalyst which has the composition as ${{H}_{2}}/Pd,BaS{{O}_{4}}$
Therefore the benzoyl chloride formed undergoes reduction to yield the desired product on treatment with Lindlar’s catalyst. This step is called Rosenmund's reduction.
This is as shown below,
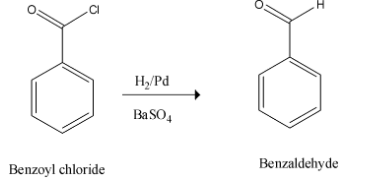
Thus, the whole of the reaction can be given in a single step. This is depicted as given below,
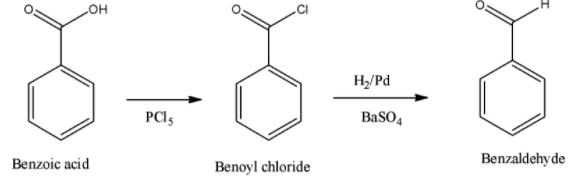
Therefore, the correct answer is benzoic acid is converted to benzaldehyde by chlorination and then followed by the reduction reaction which is a named reduction reaction.
Note: The alternate method for this reaction can also be done by slightly changing the reaction conditions like instead of phosphorus pentachloride, thionyl chloride can also be used and Lindlar’s catalyst can be replaced by $LiAl{H_4}$ followed by PCC or PDC and dot be confused when such questions are asked.
Complete step by step solution:
In our previous sections of organic chemistry we have studied the reduction reaction which involves a named reaction called Rosenmund's reduction.
- Initially the reaction is followed via conversion of benzoic acid to benzoyl chloride on treatment with phosphorus pentachloride.
- This reagent converts an acidic group to the chloride group which is called a chlorination reaction. Therefore, this reaction is as shown below

Now, after this conversion, since -Cl group is a better leaving group it easily undergoes a reduction reaction in the presence of Lindlar’s catalyst which has the composition as ${{H}_{2}}/Pd,BaS{{O}_{4}}$
Therefore the benzoyl chloride formed undergoes reduction to yield the desired product on treatment with Lindlar’s catalyst. This step is called Rosenmund's reduction.
This is as shown below,

Thus, the whole of the reaction can be given in a single step. This is depicted as given below,

Therefore, the correct answer is benzoic acid is converted to benzaldehyde by chlorination and then followed by the reduction reaction which is a named reduction reaction.
Note: The alternate method for this reaction can also be done by slightly changing the reaction conditions like instead of phosphorus pentachloride, thionyl chloride can also be used and Lindlar’s catalyst can be replaced by $LiAl{H_4}$ followed by PCC or PDC and dot be confused when such questions are asked.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




