
Convert: Bromobenzene into methyl benzene. Write the name of the reaction.
Answer
527.4k+ views
Hint: Conversion of Bromobenzene into methylbenzene is a two-step process. Bromobenzene gets converted to methylbenzene by the reaction of Bromobenzene with methyl bromide with the formation of free radicals in between.
Complete step by step answer:
We know that Benzene having ${C_6}{H_6}$ as its molecular formula is an organic compound that can be easily converted into several compounds in the presence of specific reagents. Now, the conversion of Bromobenzene to methyl benzene can be done by a named reaction, that is, Wurtz-Fittig reaction. Let’s have a look at its mechanism first. Mechanism of Wurtz-Fittig reaction can be explained by radical mechanism. In this reaction, sodium atom is used which acts as a moderator in the formation of aryl or alkyl radical which further reacts to form substituted aromatic compounds.
Mechanism:
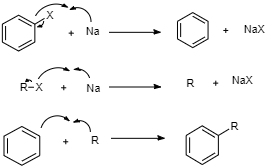
Here, in the first step, first the halide bond will cleave from the halide benzene, and then that halide will react with $Na$ and then as result benzene and sodium halide will be formed.
In the second step, there will be a bond cleavage of $R - X$ and then that halide will react with sodium which is acting as a moderator and then free radical $R$ and $NaX$ will be formed.
And finally, in the last step, benzene from the first step and alkyl halide from the second step will react together, and then finally there will be a formation of alkylbenzene.
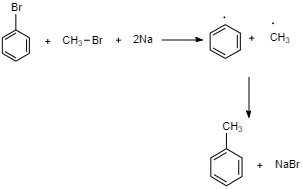
Now, let’s look at the conversion of Bromobenzene to methyl benzene.
Note:
In the conversion process, Wurtz-Fittig reaction is used, where sodium is used as a moderator which leads to the formation of radicals, and further our final product is formed.
Complete step by step answer:
We know that Benzene having ${C_6}{H_6}$ as its molecular formula is an organic compound that can be easily converted into several compounds in the presence of specific reagents. Now, the conversion of Bromobenzene to methyl benzene can be done by a named reaction, that is, Wurtz-Fittig reaction. Let’s have a look at its mechanism first. Mechanism of Wurtz-Fittig reaction can be explained by radical mechanism. In this reaction, sodium atom is used which acts as a moderator in the formation of aryl or alkyl radical which further reacts to form substituted aromatic compounds.
Mechanism:

Here, in the first step, first the halide bond will cleave from the halide benzene, and then that halide will react with $Na$ and then as result benzene and sodium halide will be formed.
In the second step, there will be a bond cleavage of $R - X$ and then that halide will react with sodium which is acting as a moderator and then free radical $R$ and $NaX$ will be formed.
And finally, in the last step, benzene from the first step and alkyl halide from the second step will react together, and then finally there will be a formation of alkylbenzene.
Now, let’s look at the conversion of Bromobenzene to methyl benzene.

Note:
In the conversion process, Wurtz-Fittig reaction is used, where sodium is used as a moderator which leads to the formation of radicals, and further our final product is formed.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




