
Convert Chlorobenzene to p−chloroaniline.
Answer
565.2k+ views
Hint:In order to answer this question, you must recall the concepts and reaction of halo-benzenes. Use the correct steps for conversion of Chlorobenzene into p-chloroaniline. For further explanation of the process, refer to the solution.
Complete answer:
Chlorobenzene is a colorless, flammable liquid with an aromatic, almond-like odor. Some of it will dissolve in water, but it readily evaporates into air. It does not occur naturally in the environment. Chlorobenzene production in the United States has declined by more than $60\% $ from its peak in $1960$. It was used in the past to make other chemicals, such as phenol and DDT. Now chlorobenzene is used as a solvent for some pesticide formulations, to degrease automobile parts, and as a chemical intermediate to make several other chemicals.
The following describe the conversion process:
Step 1: Firstly, we have to treat Chlorobenzene with $HN{O_3}$ , ${H_2}S{O_4}$ , It gives para chloronitrobenzene .
Step 2: Now in the second step, p-chloronitrobenzene is further treated with $Sn/HCl$ , known as Reduction gives p-chloroaniline.
The complete reaction is:
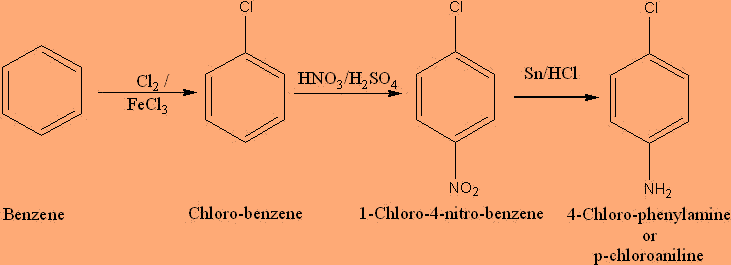
And hence, we got our required product.
Note:p-Chloroaniline is used in the industrial production of pesticides, drugs, and dyestuffs. It is a precursor to the widely used antimicrobial and bactericide chlorhexidine and is used in the manufacture of pesticides, including pyraclostrobin, anilofos, monolinuron, and chlorphthalim. It can be also used in the manufacture of some benzodiazepine drugs as well as the antihistamine dorastine, the antiarrhythmic and the antifungal ontianil. It can exhibit antimicrobial action against some bacteria and molds.
Complete answer:
Chlorobenzene is a colorless, flammable liquid with an aromatic, almond-like odor. Some of it will dissolve in water, but it readily evaporates into air. It does not occur naturally in the environment. Chlorobenzene production in the United States has declined by more than $60\% $ from its peak in $1960$. It was used in the past to make other chemicals, such as phenol and DDT. Now chlorobenzene is used as a solvent for some pesticide formulations, to degrease automobile parts, and as a chemical intermediate to make several other chemicals.
The following describe the conversion process:
Step 1: Firstly, we have to treat Chlorobenzene with $HN{O_3}$ , ${H_2}S{O_4}$ , It gives para chloronitrobenzene .
Step 2: Now in the second step, p-chloronitrobenzene is further treated with $Sn/HCl$ , known as Reduction gives p-chloroaniline.
The complete reaction is:

And hence, we got our required product.
Note:p-Chloroaniline is used in the industrial production of pesticides, drugs, and dyestuffs. It is a precursor to the widely used antimicrobial and bactericide chlorhexidine and is used in the manufacture of pesticides, including pyraclostrobin, anilofos, monolinuron, and chlorphthalim. It can be also used in the manufacture of some benzodiazepine drugs as well as the antihistamine dorastine, the antiarrhythmic and the antifungal ontianil. It can exhibit antimicrobial action against some bacteria and molds.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




