
How would you convert ethyne into benzene?
Answer
583.2k+ views
Hint:
We know that various organic compounds can be used as precursors to prepare other compounds by using suitable reagents and reaction conditions. Alkyne also shows polymerization reactions.
Complete step by step solution
We can classify hydrocarbons basically into three categories namely alkanes, alkenes and alkynes.
Alkanes consists of only single covalent bonds between the carbons. However, alkenes and alkynes have double and triple bonds between the carbons, respectively that might be in addition to single bonds. The difference in bonding renders them with different properties, physical as well as chemical. Here, we will have a look at the chemical reactions of alkynes for our given reactant belonging to that class only.
Alkynes contain $ - {\rm{C}} \equiv {\rm{C}} - $ bond and as it is evident, these two carbons have only two bond pairs which means that they are ${\rm{sp}}$ hybridized unlike alkanes and alkenes in which carbons are \[{\rm{s}}{{\rm{p}}^{\rm{3}}}\;{\rm{and}}\;{\rm{s}}{{\rm{p}}^{\rm{2}}}\] hybridized respectively. The higher $\left( {{\rm{50\% }}} \right)\;{\rm{s}}$ character makes the two $ - {\rm{C}} \equiv {\rm{C}} - $ more electronegative and ${\rm{C}} - {\rm{H}}$ bond more polar to such extent that the attached ${\rm{H}}$ become acidic in nature.
We can now discuss the chemical reactions of alkyne based on these properties as which are mainly of substitution, addition and polymerization types. Here we will take the polymerization reactions of alkynes which can be either linear or cyclic.
- Linear polymerization of ethyne can be shown as follows:

It is quite significant in the polymer industry for this polymer is a good electric conductor under proper conditions and thus can be used for this.
- Cyclic polymerization of ethyne can be shown as follows:
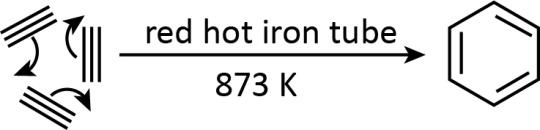
As we can see how an aliphatic compound (ethyne) can give an aromatic one (benzene) making it another important reaction of ethyne for synthetic purposes.
Hence, we can convert ethyne into benzene by its cyclic polymerization.
Note:
We know there are many different synthetic schemes that can be used with the same initial reactant giving the same final product but we have to look for an efficient one with the least number of steps.
We know that various organic compounds can be used as precursors to prepare other compounds by using suitable reagents and reaction conditions. Alkyne also shows polymerization reactions.
Complete step by step solution
We can classify hydrocarbons basically into three categories namely alkanes, alkenes and alkynes.
Alkanes consists of only single covalent bonds between the carbons. However, alkenes and alkynes have double and triple bonds between the carbons, respectively that might be in addition to single bonds. The difference in bonding renders them with different properties, physical as well as chemical. Here, we will have a look at the chemical reactions of alkynes for our given reactant belonging to that class only.
Alkynes contain $ - {\rm{C}} \equiv {\rm{C}} - $ bond and as it is evident, these two carbons have only two bond pairs which means that they are ${\rm{sp}}$ hybridized unlike alkanes and alkenes in which carbons are \[{\rm{s}}{{\rm{p}}^{\rm{3}}}\;{\rm{and}}\;{\rm{s}}{{\rm{p}}^{\rm{2}}}\] hybridized respectively. The higher $\left( {{\rm{50\% }}} \right)\;{\rm{s}}$ character makes the two $ - {\rm{C}} \equiv {\rm{C}} - $ more electronegative and ${\rm{C}} - {\rm{H}}$ bond more polar to such extent that the attached ${\rm{H}}$ become acidic in nature.
We can now discuss the chemical reactions of alkyne based on these properties as which are mainly of substitution, addition and polymerization types. Here we will take the polymerization reactions of alkynes which can be either linear or cyclic.
- Linear polymerization of ethyne can be shown as follows:

It is quite significant in the polymer industry for this polymer is a good electric conductor under proper conditions and thus can be used for this.
- Cyclic polymerization of ethyne can be shown as follows:

As we can see how an aliphatic compound (ethyne) can give an aromatic one (benzene) making it another important reaction of ethyne for synthetic purposes.
Hence, we can convert ethyne into benzene by its cyclic polymerization.
Note:
We know there are many different synthetic schemes that can be used with the same initial reactant giving the same final product but we have to look for an efficient one with the least number of steps.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




