
How will you convert glycerol to acrolein?
Answer
558k+ views
Hint: When fats are heated strongly in the presence of a dehydrating agent such as potassium bisulphate, the glycerol portion of the molecule dehydrates to form the unsaturated aldehyde, this is how we obtain acrolein from glycerol. This is a normal day to day phenomena taking place in our kitchens but we fail to notice it.
Complete step-by-step answer:Glycerol when heated alone or with dehydrating agents such as potassium hydrogen sulphate or phosphorous pentoxide or concentrated sulphuric, the $\beta - $elimination reaction takes place to give acrolein, which has a characteristic bad smell.
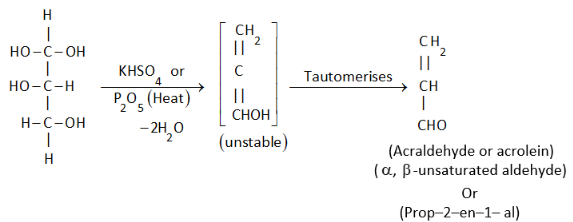
This is the acrolein test.
Additional Information: Acrolein test is used to detect the presence of glycerol or fat.
Glycerol is an essential sugar alcohol for many living things. For one, it is a component of lipids such as triglycerides and phospholipids along with fatty acids. Glycerol forms glycerides that can serve as an energy fuel. Triglycerides, for instance, is a major component of animal fats and vegetable oils.
Acrolein was first discovered by Jons Jacobs Berzelius in,\[1839\]while working on the thermal degradation products of glycerol, a material then well known for its use in manufacturing soaps. Its name is derived from the words acrid and oleum which mean pungent smelling and having oil like consistency. It in its early days after discovery was highly used in production of acrylic acid and acrylic plastics.
Note:The unstable tautomer formed during the reaction is really short lived and tautomerizes into a more stable form as soon as it is formed during the reaction, also the formation of acrolein is indicated by its characteristic bad smell which helps in easy detection of the compound in laboratories.
Complete step-by-step answer:Glycerol when heated alone or with dehydrating agents such as potassium hydrogen sulphate or phosphorous pentoxide or concentrated sulphuric, the $\beta - $elimination reaction takes place to give acrolein, which has a characteristic bad smell.

This is the acrolein test.
Additional Information: Acrolein test is used to detect the presence of glycerol or fat.
Glycerol is an essential sugar alcohol for many living things. For one, it is a component of lipids such as triglycerides and phospholipids along with fatty acids. Glycerol forms glycerides that can serve as an energy fuel. Triglycerides, for instance, is a major component of animal fats and vegetable oils.
Acrolein was first discovered by Jons Jacobs Berzelius in,\[1839\]while working on the thermal degradation products of glycerol, a material then well known for its use in manufacturing soaps. Its name is derived from the words acrid and oleum which mean pungent smelling and having oil like consistency. It in its early days after discovery was highly used in production of acrylic acid and acrylic plastics.
Note:The unstable tautomer formed during the reaction is really short lived and tautomerizes into a more stable form as soon as it is formed during the reaction, also the formation of acrolein is indicated by its characteristic bad smell which helps in easy detection of the compound in laboratories.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




