
How do you convert:
i) Chlorobenzene to biphenyl
ii) Propene to 1-iodopropane
iii) 2-bromobutane to but-2-ene
Answer
524.9k+ views
Hint: As we know fittig reaction is also written as wurtz-fittig reaction. Fittig reaction happens between alkyl halides, aryl halides and sodium metal in presence of dry ether. We can get substituted aromatic compounds from this reaction.
Complete step by step answer:
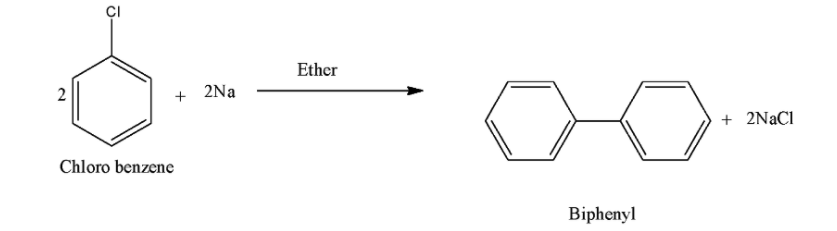
(i) Chloro benzene to biphenyl:
As we know we use Fittig reaction to convert chloro benzene to biphenyl. Two moles of sodium reacts with chlorobenzene in presence of ether and form biphenyl. The reaction for the formation of biphenyl from chloro benzene is as follows:
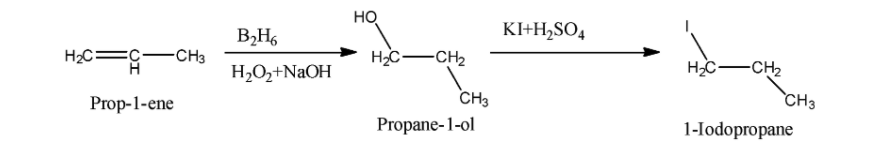
(ii) Propene to 1-iodopropane:
We know that Propene reacts with diborane and hydrogen peroxide in presence of sodium hydroxide and form propan -1-ol. This process is also called hydroboration oxidation. After formation of propane -1-ol, propane -1-ol reacts with potassium iodide in presence of sulfuric acid and form 1-iodopropane. The reaction of formation of 1-iodopropane is as follows:
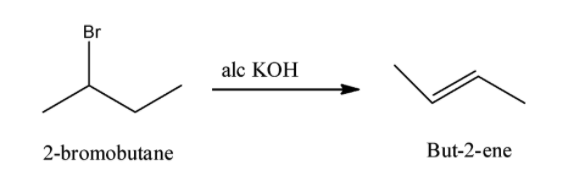
(iii) 2-bromobutane to but-2-ene:
We know that 2-bromobutane reacts with alcoholic KOH and form but-2-ene. This reaction is also called dehydration reaction. The reaction for formation of but-2-ene is as follows:
Note:
We know that hydroboration oxidation is used to produce alcohol from alkene. It is a two step pathway. Anti-markovnikov rule is applied in hydroboration oxidation reaction. In this reaction hydroxyl group is attached to less substituted carbon. As we know dehydration reaction is change which involves loss of water molecule from reaction ion or molecule. Sulfuric acid and aluminum are common dehydrating agents.
Complete step by step answer:
(i) Chloro benzene to biphenyl:
As we know we use Fittig reaction to convert chloro benzene to biphenyl. Two moles of sodium reacts with chlorobenzene in presence of ether and form biphenyl. The reaction for the formation of biphenyl from chloro benzene is as follows:

(ii) Propene to 1-iodopropane:
We know that Propene reacts with diborane and hydrogen peroxide in presence of sodium hydroxide and form propan -1-ol. This process is also called hydroboration oxidation. After formation of propane -1-ol, propane -1-ol reacts with potassium iodide in presence of sulfuric acid and form 1-iodopropane. The reaction of formation of 1-iodopropane is as follows:

(iii) 2-bromobutane to but-2-ene:
We know that 2-bromobutane reacts with alcoholic KOH and form but-2-ene. This reaction is also called dehydration reaction. The reaction for formation of but-2-ene is as follows:

Note:
We know that hydroboration oxidation is used to produce alcohol from alkene. It is a two step pathway. Anti-markovnikov rule is applied in hydroboration oxidation reaction. In this reaction hydroxyl group is attached to less substituted carbon. As we know dehydration reaction is change which involves loss of water molecule from reaction ion or molecule. Sulfuric acid and aluminum are common dehydrating agents.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




