
How will you convert phenol to benzoic acid?
Answer
569.1k+ views
Hint: We can convert phenol to benzoic acid very easily. But, there is no direct conversion or single-step conversion of phenol to benzoic acid. We need to create some intermediates to convert phenol to benzoic acid. Try to draw structures first and then step by step to convert the structure using reagents.
Complete step by step answer:
Phenol is an aromatic organic compound with a molecular formula ${C_6}{H_5}OH$. The other compound is benzoic acid. It is an organic compound with a molecular formula ${C_6}{H_5}COOH$. It is also known as a carboxylic acid. So we need to convert phenol to benzoic acid.
Here are some basic steps of conversions of phenol to benzoic acid.
Conversion of phenol using zinc dust into benzene.
Using $\left( {C{H_3}Cl + AlC{l_3}} \right)$ where aluminium trichloride acts as a Lewis acid catalyst. This reaction is also known as the Friedel-crafts alkylation reaction.
The result obtained above is alkyl benzene which on oxidation gives benzoic acid.
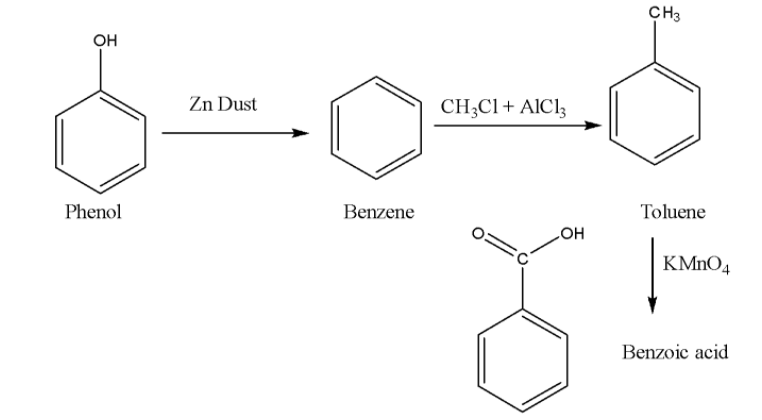
When phenol reacts with zinc dust at high temperature the phenol converts to phenoxide ion and the proton. This proton accepts an electron from zinc and results in the formation of zinc oxide. After that phenoxide ion converts into benzene.
The next step is the Friedel crafts alkylation reaction of benzene. It is the best way of attaching a hydrocarbon-based group to a benzene ring. The attacking reagent used for the reaction is alkyl halide and ammonium trichloride.
It is observed that reacting an alkylbenzene with the reducing agent potassium permanganate $KMn{O_4}$ gives the oxidized product of alkylbenzene that is benzoic acid.
Note:
The reaction of toluene or alkyl benzene with potassium permanganate only works if there is hydrogen attached to the carbon as it oxidizes the only hydrocarbon.
One of the limitations of Friedel-crafts alkylation reaction is that the halide must be either an alkyl halide. These reactions are based on the stability of carbocations. So the most stable carbocation forms will form the product.
Complete step by step answer:
Phenol is an aromatic organic compound with a molecular formula ${C_6}{H_5}OH$. The other compound is benzoic acid. It is an organic compound with a molecular formula ${C_6}{H_5}COOH$. It is also known as a carboxylic acid. So we need to convert phenol to benzoic acid.

Here are some basic steps of conversions of phenol to benzoic acid.
Conversion of phenol using zinc dust into benzene.
Using $\left( {C{H_3}Cl + AlC{l_3}} \right)$ where aluminium trichloride acts as a Lewis acid catalyst. This reaction is also known as the Friedel-crafts alkylation reaction.
The result obtained above is alkyl benzene which on oxidation gives benzoic acid.

When phenol reacts with zinc dust at high temperature the phenol converts to phenoxide ion and the proton. This proton accepts an electron from zinc and results in the formation of zinc oxide. After that phenoxide ion converts into benzene.
The next step is the Friedel crafts alkylation reaction of benzene. It is the best way of attaching a hydrocarbon-based group to a benzene ring. The attacking reagent used for the reaction is alkyl halide and ammonium trichloride.
It is observed that reacting an alkylbenzene with the reducing agent potassium permanganate $KMn{O_4}$ gives the oxidized product of alkylbenzene that is benzoic acid.
Note:
The reaction of toluene or alkyl benzene with potassium permanganate only works if there is hydrogen attached to the carbon as it oxidizes the only hydrocarbon.
One of the limitations of Friedel-crafts alkylation reaction is that the halide must be either an alkyl halide. These reactions are based on the stability of carbocations. So the most stable carbocation forms will form the product.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




