
How will you convert phenol to phenolphthalein?
Answer
594.9k+ views
Hint: The conversion is condensation of phthalic anhydride with two equivalents of phenol under acidic conditions. The structure of phenolphthalein has three benzene rings, one carbonyl atom, one oxygen atom and two -OH groups. It is a universal indicator, which is used to determine whether a solution is acidic or basic in nature.
Complete step by step answer:
* It is well known to us that Phenolphthalein is used as an indicator in acid – base titrations.
* Because of its colour changing property Phenolphthalein with chemical formula \[{{C}_{20}}{{H}_{14}}{{O}_{4}}\] is a component of universal indicator, together with methyl red, bromothymol blue and thymol blue.
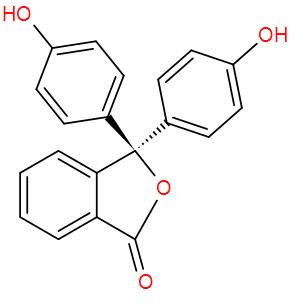
* We can carry out synthesis of phenolphthalein by condensation of phenol with phthalic anhydride in the presence of an acid (${{H}_{2}}S{{O}_{4}}$).
* Phenols are heated with phthalic anhydride and conc. ${{H}_{2}}S{{O}_{4}}$ to form phenolphthalein. Reaction is as follows:
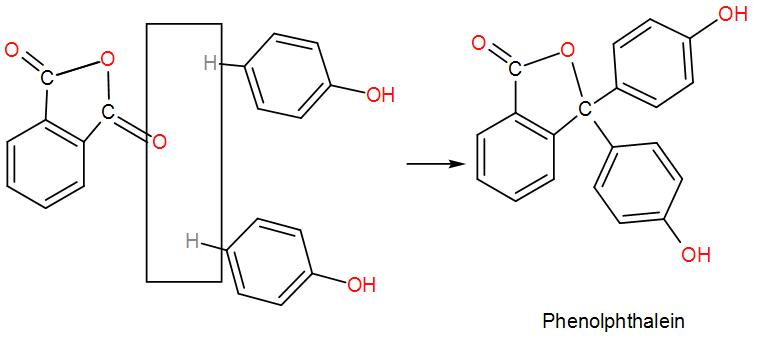
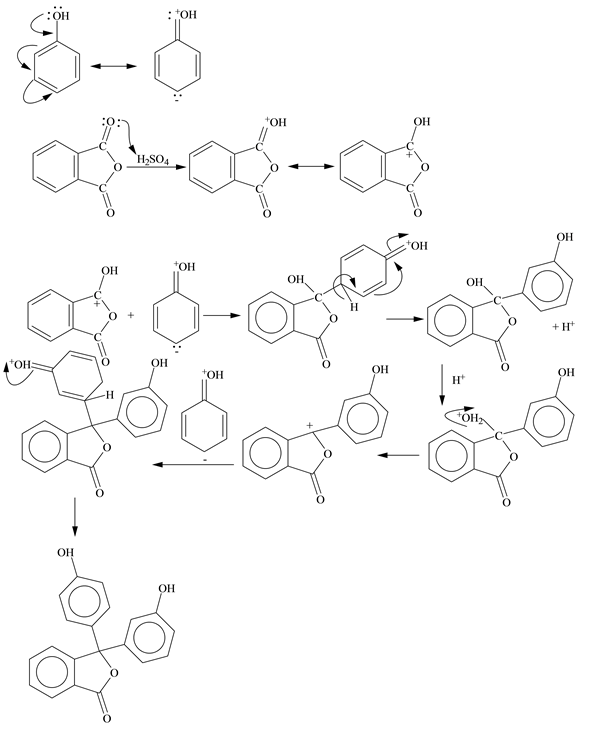
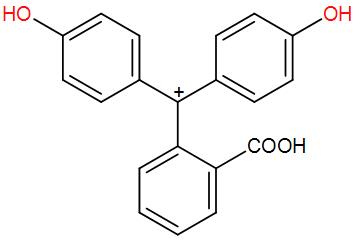
* For better understanding see the mechanism given below:
* We use phenolphthalein as an indicator because of its ability to show four different states due to change in pH.
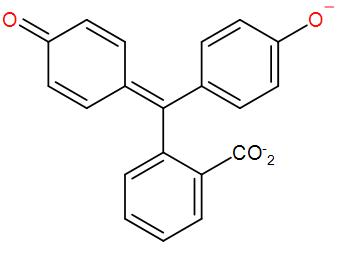
* In the basic medium it changes from colourless to pink because of the formation of phenolate ion (anionic form of phenol).
* In strongly basic medium we observe that colour change slowly fades and at pH greater than 13 it becomes completely colourless
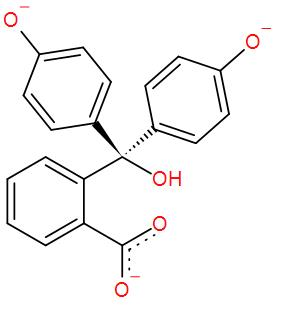
* While it remains colourless in slightly acidic or in neutral medium
* We can also observe the colour change from colourless to orange in strongly acidic medium (pH less than 1)
Note:
(i) This reaction is electrophilic substitution reaction.
(ii) The electrophilic carbon attack at para position.
(iii) Phthalic anhydride can be easily formed from phthalic acid.
Complete step by step answer:
* It is well known to us that Phenolphthalein is used as an indicator in acid – base titrations.
* Because of its colour changing property Phenolphthalein with chemical formula \[{{C}_{20}}{{H}_{14}}{{O}_{4}}\] is a component of universal indicator, together with methyl red, bromothymol blue and thymol blue.
* We can carry out synthesis of phenolphthalein by condensation of phenol with phthalic anhydride in the presence of an acid (${{H}_{2}}S{{O}_{4}}$).
* Phenols are heated with phthalic anhydride and conc. ${{H}_{2}}S{{O}_{4}}$ to form phenolphthalein. Reaction is as follows:

* For better understanding see the mechanism given below:

* We use phenolphthalein as an indicator because of its ability to show four different states due to change in pH.
* In the basic medium it changes from colourless to pink because of the formation of phenolate ion (anionic form of phenol).

* In strongly basic medium we observe that colour change slowly fades and at pH greater than 13 it becomes completely colourless

* While it remains colourless in slightly acidic or in neutral medium

* We can also observe the colour change from colourless to orange in strongly acidic medium (pH less than 1)

Note:
(i) This reaction is electrophilic substitution reaction.
(ii) The electrophilic carbon attack at para position.
(iii) Phthalic anhydride can be easily formed from phthalic acid.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




