
How to convert styrene to benzoic acid?
Answer
516.9k+ views
Hint: Styrene is an organic compound with the chemical formula of ${C_6}{H_5}CH = C{H_2}$. It is a derivative of benzene and appears to be a colourless oily liquid and turns yellow when aged. The compound gets evaporated very easily and has a sweet smell but in high concentration, the pleasant smell becomes less. Styrene also acts as a precursor to polystyrene and several other copolymers.
Complete answer: Benzoic acid is an organic compound with the chemical formula of ${C_6}{H_5}COOH$ and consists of a carboxyl group attached to a benzene ring. Benzoic acid is an aromatic carboxylic acid and exists as a crystalline, colourless solid under normal conditions.
Styrene is also known as phenyl ethane and can be converted to benzoic acid by reacting with either $KMn{O_4}$ or diluted ${H_2}S{O_4}$ in the presence of heat. The end product of the reaction gives benzoic acid and carbon dioxide and ${H_2}O$.
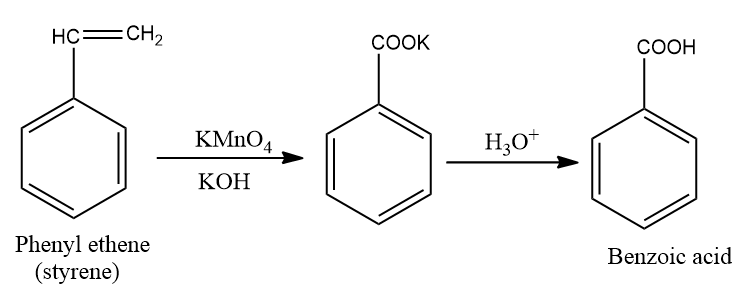
Fig: Reaction showing the formation of benzoic acid from styrene.
Styrene is treated with potassium permanganate in aqueous solution of potassium hydroxide which gives intermediate potassium benzoate. Potassium benzoate is then treated with ${H_3}{O^ + }$ which gives benzoic acid.
Note:
Benzoic acid is soluble in water, benzene, carbon tetrachloride, acetone and alcohol. It is primarily used in the industrial production of aromatic phenol compounds and it is done by the process called oxidative decarboxylation. Benzoic acid and its salts are widely used in the food industries as food preservatives.
Benzoic acid can also be prepared by using oxygen gas for the partial oxidation of toluene and this process uses manganese or cobalt naphthenate as catalysts.
Complete answer: Benzoic acid is an organic compound with the chemical formula of ${C_6}{H_5}COOH$ and consists of a carboxyl group attached to a benzene ring. Benzoic acid is an aromatic carboxylic acid and exists as a crystalline, colourless solid under normal conditions.
Styrene is also known as phenyl ethane and can be converted to benzoic acid by reacting with either $KMn{O_4}$ or diluted ${H_2}S{O_4}$ in the presence of heat. The end product of the reaction gives benzoic acid and carbon dioxide and ${H_2}O$.

Fig: Reaction showing the formation of benzoic acid from styrene.
Styrene is treated with potassium permanganate in aqueous solution of potassium hydroxide which gives intermediate potassium benzoate. Potassium benzoate is then treated with ${H_3}{O^ + }$ which gives benzoic acid.
Note:
Benzoic acid is soluble in water, benzene, carbon tetrachloride, acetone and alcohol. It is primarily used in the industrial production of aromatic phenol compounds and it is done by the process called oxidative decarboxylation. Benzoic acid and its salts are widely used in the food industries as food preservatives.
Benzoic acid can also be prepared by using oxygen gas for the partial oxidation of toluene and this process uses manganese or cobalt naphthenate as catalysts.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




