
How will you convert the following?
(i) Phenol to salicylic acid
(ii) Ethylene to ethanol
(iii) Acetaldehyde to propan-2-ol
(iv) Acetic acid to acetaldehyde
(v) Methyl chloride to acetone
(vi) Benzoic acid to aniline
(vii) Aniline to benzene
(viii) Aniline to benzoic acid.
Answer
548.1k+ views
Hint: In order to answer the above question, we must be aware of the basic chemical reaction that is taking place in the given compound. We should also be aware of the reagents that are used in a given chemical reaction.
Complete step by step answer:
Let us move onto the questions given:
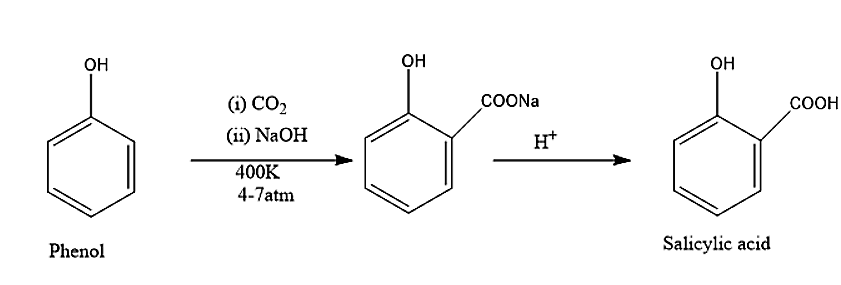
(i) The reaction in which the phenol is converted into salicylic acid is known as Kolbe’s reaction. In this reaction phenol will react with the carbon dioxide in presence of a strong base like sodium hydroxide, to give a product of salicylic acid or o-hydroxy benzoic acid. the temperature at which the reaction is taking place is 400K and the pressure is 4 – 7 atm. Kolbe’s reaction is given below:
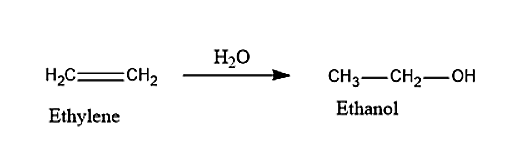
(ii) ethanol can be obtained from ethylene by passing steam. The conversion of ethylene into ethanol will take place by the process of hydrolysis. The given reaction is an exothermic reaction and the reaction is given below:
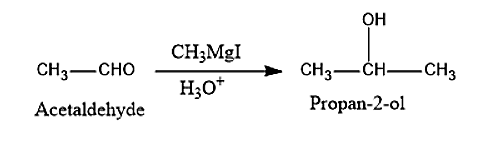
(iii) The Acetaldehyde when treated with Grignard reagent like Methyl magnesium iodide, i.e., \[C{H_3}MgI\] followed by acid hydrolysis will lead to the formation of propan-2-ol.
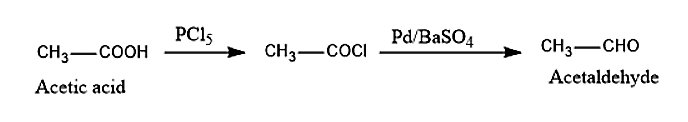
(iv) In order to convert acetic acid to acetaldehyde, we must first convert the acetic acid to acyl chloride by reacting with \[PC{l_5}\]. then this acyl chloride is converted into acetaldehyde by the Rosenmund reduction method by using Bayer’s reagent \[Pd/BaS{O_4}\]. We can write the reaction as
(v) Methyl chloride can be converted into acetone by reacting with formaldehyde. The reaction of converting Methyl chloride is given below:
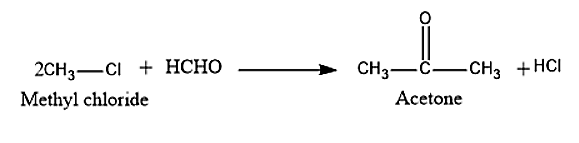
(vi) In order to convert benzoic acid to aniline, first we have to react benzoic acid with \[SOC{l_2}\] to obtain Benzoyl chloride. This benzoyl chloride with ammonia to get Benzamide. This Benzamide is further reacted with \[B{r_2}\] and it will undergo Hoffman Bromamide degradation to get Aniline.
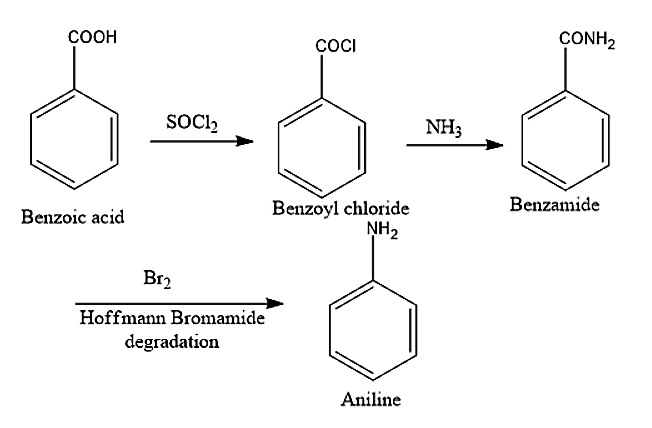
(vii) Aniline can be converted to Benzene, by first reacting Aniline with sodium nitrite with Hydrochloric acid to obtain benzene diazonium chloride. This is further heated with \[{H_3}P{O_2}\] to obtain Benzene.
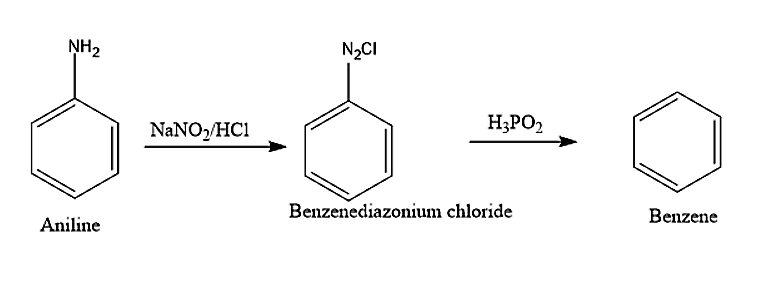
(viii) Aniline on reacting with Sodium nitrite and hydrochloric acid will produce Benzene diazonium chloride. This benzene diazonium chloride will react with \[CuCN/KCN\]to produce Benzonitrile. Benzonitrile on hydrolysis will produce benzoic acid.
Note: We should never forget to write about the reagents used in certain chemical reactions. The reaction will be complete only when the reagents are written above the arrow. We can also mention the conditions like temperature and pressure in the arrow.
Complete step by step answer:
Let us move onto the questions given:
(i) The reaction in which the phenol is converted into salicylic acid is known as Kolbe’s reaction. In this reaction phenol will react with the carbon dioxide in presence of a strong base like sodium hydroxide, to give a product of salicylic acid or o-hydroxy benzoic acid. the temperature at which the reaction is taking place is 400K and the pressure is 4 – 7 atm. Kolbe’s reaction is given below:

(ii) ethanol can be obtained from ethylene by passing steam. The conversion of ethylene into ethanol will take place by the process of hydrolysis. The given reaction is an exothermic reaction and the reaction is given below:

(iii) The Acetaldehyde when treated with Grignard reagent like Methyl magnesium iodide, i.e., \[C{H_3}MgI\] followed by acid hydrolysis will lead to the formation of propan-2-ol.

(iv) In order to convert acetic acid to acetaldehyde, we must first convert the acetic acid to acyl chloride by reacting with \[PC{l_5}\]. then this acyl chloride is converted into acetaldehyde by the Rosenmund reduction method by using Bayer’s reagent \[Pd/BaS{O_4}\]. We can write the reaction as

(v) Methyl chloride can be converted into acetone by reacting with formaldehyde. The reaction of converting Methyl chloride is given below:

(vi) In order to convert benzoic acid to aniline, first we have to react benzoic acid with \[SOC{l_2}\] to obtain Benzoyl chloride. This benzoyl chloride with ammonia to get Benzamide. This Benzamide is further reacted with \[B{r_2}\] and it will undergo Hoffman Bromamide degradation to get Aniline.

(vii) Aniline can be converted to Benzene, by first reacting Aniline with sodium nitrite with Hydrochloric acid to obtain benzene diazonium chloride. This is further heated with \[{H_3}P{O_2}\] to obtain Benzene.

(viii) Aniline on reacting with Sodium nitrite and hydrochloric acid will produce Benzene diazonium chloride. This benzene diazonium chloride will react with \[CuCN/KCN\]to produce Benzonitrile. Benzonitrile on hydrolysis will produce benzoic acid.

Note: We should never forget to write about the reagents used in certain chemical reactions. The reaction will be complete only when the reagents are written above the arrow. We can also mention the conditions like temperature and pressure in the arrow.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




