
Correct order of resonance stabilisation in $\text{ }{{\text{H}}_{\text{2}}}\text{C}{{\text{O}}_{\text{3 }}}$and $\text{ CO}_{3}^{2-}\text{ }$ is:
(A) $\text{ CO}_{3}^{2-}\text{ }>\text{ }{{\text{H}}_{\text{2}}}\text{C}{{\text{O}}_{\text{3}}}\text{ }$
(B) $\text{ CO}_{3}^{2-}\text{ }<\text{ }{{\text{H}}_{\text{2}}}\text{C}{{\text{O}}_{\text{3}}}\text{ }$
(C) $\text{ CO}_{3}^{2-}\text{ = }{{\text{H}}_{\text{2}}}\text{C}{{\text{O}}_{\text{3}}}\text{ }$
(D) None of these
Answer
564.9k+ views
Hint: The resonance is a delocalisation of electrons within a molecule. It results in the resonating structure which contributes towards the stability of the molecule. It is found that, number of resonating structures are directly related to the stability of molecules. Higher the number of resonating structures greater is the stability of molecules.
Complete Solution :
Resonance is a process of delocalisation of electrons within a molecule. It involves the construction of multiple Lewis dot structures when combined gives the full electronic structure of molecules.
The delocalisation of electrons lowers the potential energy of the molecule and stabilises the structure. More the number of resonating structures, higher is the drop in potential energy and more stable is the structure of molecules.
- Let’s consider the example of carbonic acid and carbonate ion.The carbonic acid have a general molecular formula as $\text{ }{{\text{H}}_{\text{2}}}\text{C}{{\text{O}}_{\text{3 }}}$ .Here the two oxygen atoms of carbonate group are protonated. The carbonic acid does not have free electrons or pi-electrons which can take part in delocalisation. Therefore, carbonic acid exists in only a single form.
It does not have a resonating structure. It is as shown below:
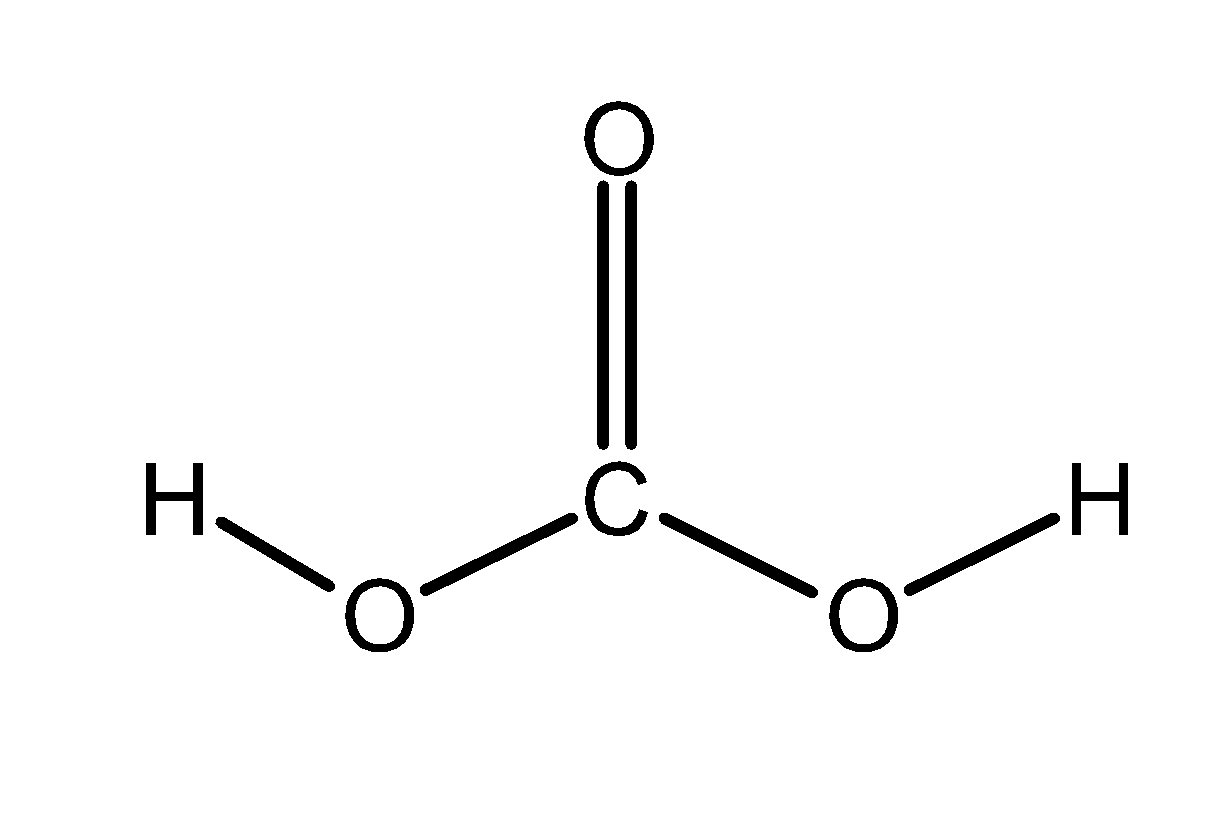
The carbonate ion has a molecular formula as $\text{ CO}_{3}^{2-}\text{ }$ .the negative charge or the lone pair of electron on one oxygen atom and pi-bonded electrons takes part in resonating structure. The lone pair and pi-electrons shift to give resonating structure. These are as shown below:
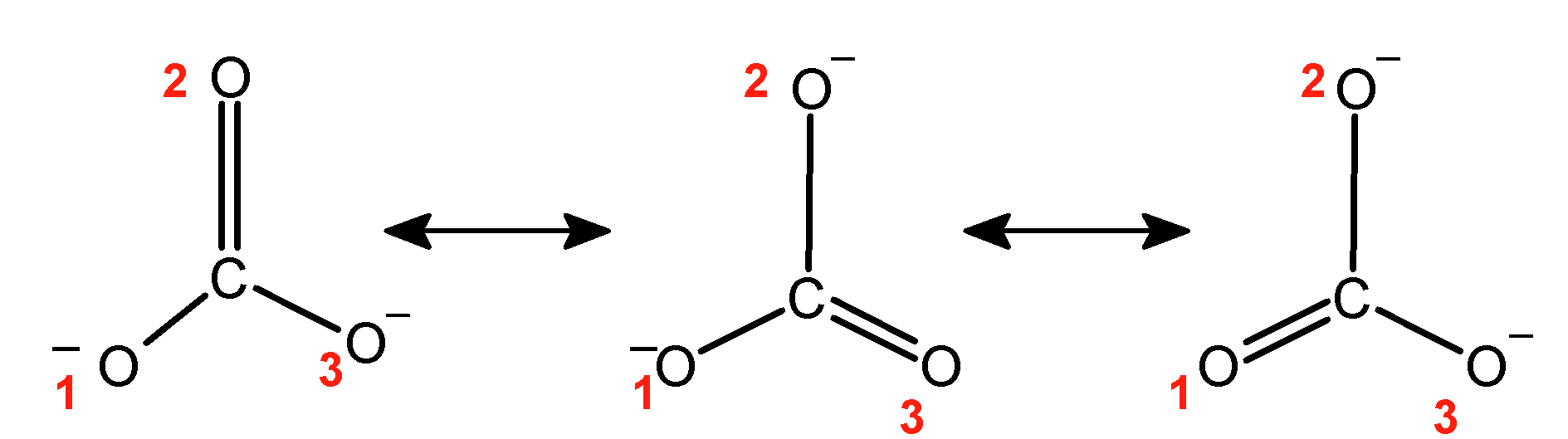
Here carbonyl carbon is bonded to three oxygen atoms .Each oxygen atom acquires the double bond. Thus carbonate ions have three resonating structures.
We know that stability of structure is directly related to the number of resonating structures. Thus resonance stabilisation of $\text{ CO}_{3}^{2-}\text{ }$ ion is greater than $\text{ }{{\text{H}}_{\text{2}}}\text{C}{{\text{O}}_{\text{3 }}}$. That is,
$\text{ CO}_{3}^{2-}\text{ }>\text{ }{{\text{H}}_{\text{2}}}\text{C}{{\text{O}}_{\text{3}}}\text{ }$
So, the correct answer is “Option A”.
Note: Note that , when a molecule exists in two or more resonating structure then the resonating structure which have a negative charge on the electronegative charge is more stable .Here all three oxygen atoms acquires the negative charge and thus these are most stable structures.
Complete Solution :
Resonance is a process of delocalisation of electrons within a molecule. It involves the construction of multiple Lewis dot structures when combined gives the full electronic structure of molecules.
The delocalisation of electrons lowers the potential energy of the molecule and stabilises the structure. More the number of resonating structures, higher is the drop in potential energy and more stable is the structure of molecules.
- Let’s consider the example of carbonic acid and carbonate ion.The carbonic acid have a general molecular formula as $\text{ }{{\text{H}}_{\text{2}}}\text{C}{{\text{O}}_{\text{3 }}}$ .Here the two oxygen atoms of carbonate group are protonated. The carbonic acid does not have free electrons or pi-electrons which can take part in delocalisation. Therefore, carbonic acid exists in only a single form.
It does not have a resonating structure. It is as shown below:

The carbonate ion has a molecular formula as $\text{ CO}_{3}^{2-}\text{ }$ .the negative charge or the lone pair of electron on one oxygen atom and pi-bonded electrons takes part in resonating structure. The lone pair and pi-electrons shift to give resonating structure. These are as shown below:

Here carbonyl carbon is bonded to three oxygen atoms .Each oxygen atom acquires the double bond. Thus carbonate ions have three resonating structures.
We know that stability of structure is directly related to the number of resonating structures. Thus resonance stabilisation of $\text{ CO}_{3}^{2-}\text{ }$ ion is greater than $\text{ }{{\text{H}}_{\text{2}}}\text{C}{{\text{O}}_{\text{3 }}}$. That is,
$\text{ CO}_{3}^{2-}\text{ }>\text{ }{{\text{H}}_{\text{2}}}\text{C}{{\text{O}}_{\text{3}}}\text{ }$
So, the correct answer is “Option A”.
Note: Note that , when a molecule exists in two or more resonating structure then the resonating structure which have a negative charge on the electronegative charge is more stable .Here all three oxygen atoms acquires the negative charge and thus these are most stable structures.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




