
How do you count sigma and pi bonds? For example, in the Lewis structure azidothymidine there are $33$ sigma and $5$ pi bonds. How is this found?
Answer
558.6k+ views
Hint: A sigma bond is formed by the head on overlap of atomic orbitals and a pi bond is formed by side-by-side overlap of atomic orbitals. Sigma bond is stronger than a pi bond.
Complete step by step answer:
The given compound is azidothymidine. Its structure is shown below.
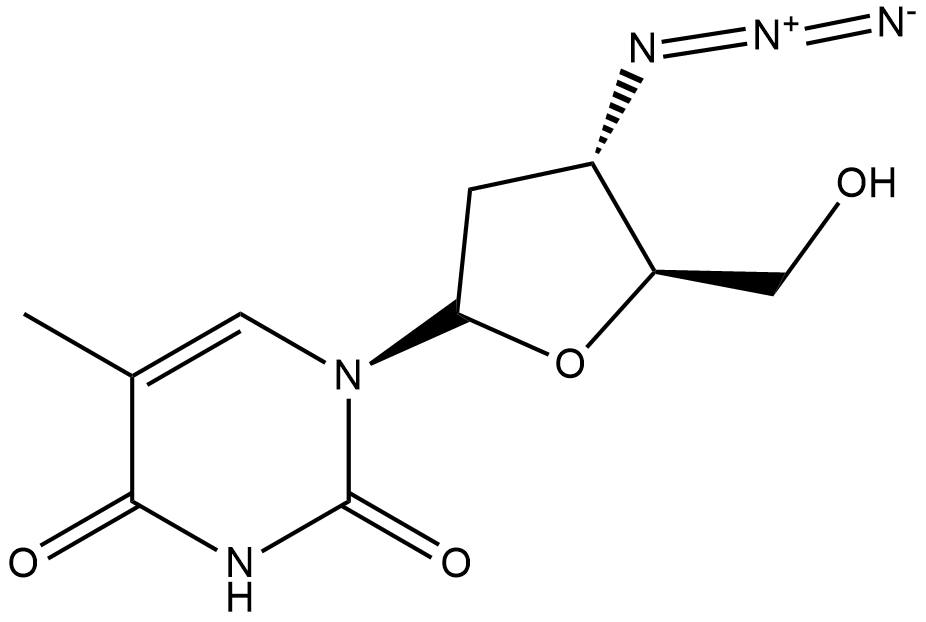
In this structure, the carbon and hydrogens are not shown separately. In order to count sigma and pi bonds we need to show all the bonds in the molecule. Hence let us draw another structure of the given molecule showing all the bonds.
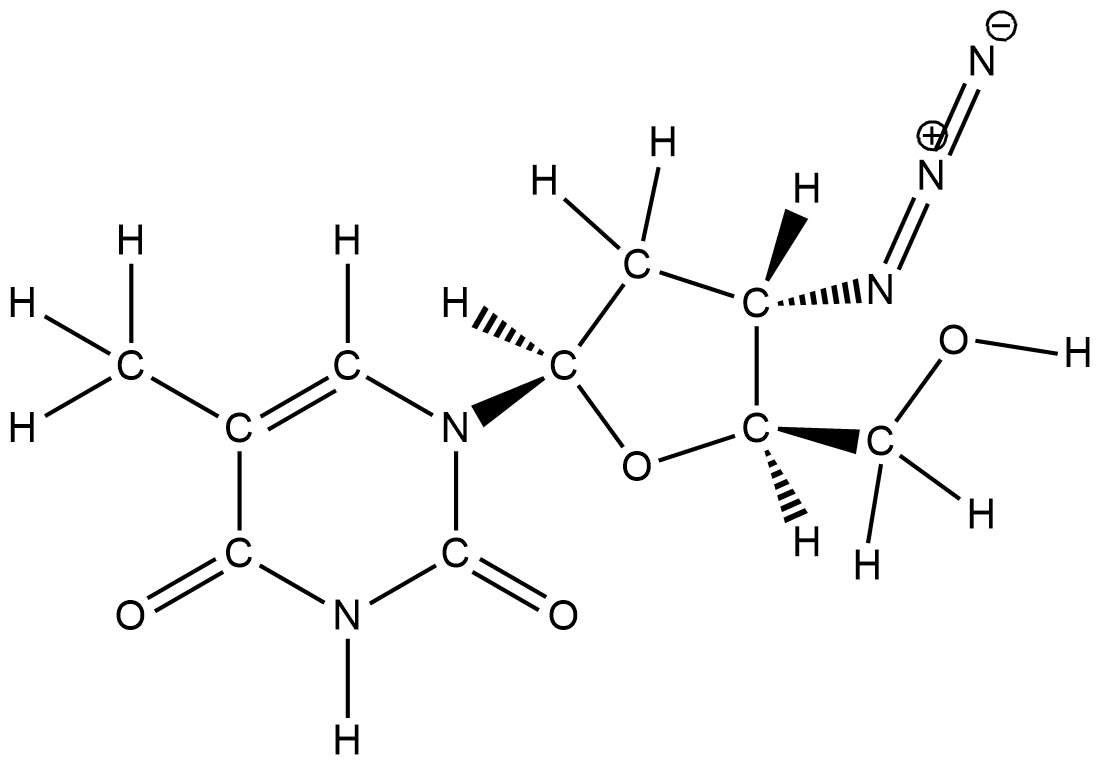
To count the number of sigma and pi bonds we need to know some points. All single bonds are formed by head on overlap of atomic orbitals. Hence all single bonds are sigma bonds. A double or triple bond consists of both sigma and pi bonds. A double bond is formed by one sigma bond and one pi bond. A triple bond is formed by one sigma bond and two pi bonds.
In the given molecule there is no triple bond. Only single bonds and double bonds are present. Hence the number of sigma bonds will be equal to the number of single bonds. By counting the bonds we get that there are $33$bonds in the molecule. Hence the number of sigma bonds is $33$ . Now let us count the number of double bonds. There are $5$ double bonds. Hence the number of pi bonds is $5$ .
Note:
The sigma bond is formed by the overlap of hybridised orbitals on the atom. But the pi bond is formed by the un-hybridised orbitals left on the atom. Pi bond is more reactive than sigma bond.
Complete step by step answer:
The given compound is azidothymidine. Its structure is shown below.

In this structure, the carbon and hydrogens are not shown separately. In order to count sigma and pi bonds we need to show all the bonds in the molecule. Hence let us draw another structure of the given molecule showing all the bonds.

To count the number of sigma and pi bonds we need to know some points. All single bonds are formed by head on overlap of atomic orbitals. Hence all single bonds are sigma bonds. A double or triple bond consists of both sigma and pi bonds. A double bond is formed by one sigma bond and one pi bond. A triple bond is formed by one sigma bond and two pi bonds.
In the given molecule there is no triple bond. Only single bonds and double bonds are present. Hence the number of sigma bonds will be equal to the number of single bonds. By counting the bonds we get that there are $33$bonds in the molecule. Hence the number of sigma bonds is $33$ . Now let us count the number of double bonds. There are $5$ double bonds. Hence the number of pi bonds is $5$ .
Note:
The sigma bond is formed by the overlap of hybridised orbitals on the atom. But the pi bond is formed by the un-hybridised orbitals left on the atom. Pi bond is more reactive than sigma bond.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




