
CsCl crystallizes in a cubic lattice that has a \[C{{l}^{-}}\] at each corner and \[C{{s}^{+}}\] at the centre of the unit cell. If \[\left( {{r}_{C{{s}^{+}}}} \right)=1.69A{}^\circ \] and \[\left( {{r}_{C{{l}^{-}}}} \right)=1.81A{}^\circ \] What is the value of edge length of the cube?
(A) 4.04
(B) 3.50
(C) 3.03
(D) 1.95
Answer
585.9k+ views
Hint: Try to recall that in CsCl structure, chloride ions have simple cubic arrangement and caesium ions occupy cubic sites. Now, by using this you can easily find the value of edge length of the cube.
Complete step by step answer:
It is known to you that the chloride ions occupy the corners of the cube and caesium ions lie in the cubic void present at the body centre of the cubic unit cell.
The coordination number of both cation and anion in CsCl structure is 8 and the total number of both cation and anion in CsCl crystal is one.
This structure is also exhibited by CsBr, CsI and many binary metallic alloys.
Calculation:
To find out the value of edge length of the cube, we need to find the relationship between interionic distance and edge length. Let ‘a’ be the edge length of the cube.
Since, both Chloride ion and caesium ion touches each other along body diagonal
Given,
\[\begin{align}
& \left( {{r}_{C{{s}^{+}}}} \right)=1.69{{A}^{{}^\circ }}\text{ } \\
& \left( {{r}_{C{{l}^{-}}}} \right)=1.81{{A}^{{}^\circ }} \\
\end{align}\]
\[\begin{align}
& 2{{r}_{C{{s}^{+}}}}+2{{r}_{C{{l}^{-}}}}=\sqrt{3}a \\
& \Rightarrow 2\times \left( {{r}_{C{{s}^{+}}}}+{{r}_{C{{l}^{-}}}} \right)=\sqrt{3}a \\
& \Rightarrow a=\frac{2\times \left( {{r}_{C{{s}^{+}}}}+{{r}_{C{{l}^{-}}}} \right)}{\sqrt{3}}=\dfrac{2\times \left( 1.69+1.81 \right)}{\sqrt{3}}=4.041A{}^\circ \\
\end{align}\]
So, the correct answer is “Option B”.
Note: It should be remembered to you that on application of high pressure, The NaCl structure changes to CsCl structure whereas on heating the CsCl structure at 760K, the CsCl structure transforms to NaCl structure.
The structure of NaCl and CsCl is given below:
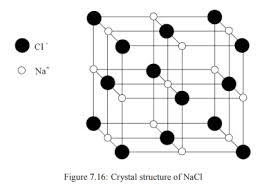
The black atoms correspond to $C{{l}^{-}}$ ions and white atoms correspond to $N{{a}^{+}}$ atoms.
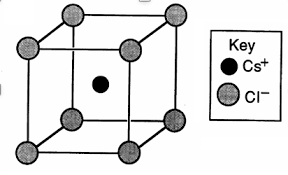
The grey atoms correspond to $C{{l}^{-}}$ ions and black atom is $C{{s}^{+}}$ ion.
Also, you should remember that CsCl is used in isopycnic centrifugation for separating various types of DNA.
Complete step by step answer:
It is known to you that the chloride ions occupy the corners of the cube and caesium ions lie in the cubic void present at the body centre of the cubic unit cell.
The coordination number of both cation and anion in CsCl structure is 8 and the total number of both cation and anion in CsCl crystal is one.
This structure is also exhibited by CsBr, CsI and many binary metallic alloys.
Calculation:
To find out the value of edge length of the cube, we need to find the relationship between interionic distance and edge length. Let ‘a’ be the edge length of the cube.
Since, both Chloride ion and caesium ion touches each other along body diagonal
Given,
\[\begin{align}
& \left( {{r}_{C{{s}^{+}}}} \right)=1.69{{A}^{{}^\circ }}\text{ } \\
& \left( {{r}_{C{{l}^{-}}}} \right)=1.81{{A}^{{}^\circ }} \\
\end{align}\]
\[\begin{align}
& 2{{r}_{C{{s}^{+}}}}+2{{r}_{C{{l}^{-}}}}=\sqrt{3}a \\
& \Rightarrow 2\times \left( {{r}_{C{{s}^{+}}}}+{{r}_{C{{l}^{-}}}} \right)=\sqrt{3}a \\
& \Rightarrow a=\frac{2\times \left( {{r}_{C{{s}^{+}}}}+{{r}_{C{{l}^{-}}}} \right)}{\sqrt{3}}=\dfrac{2\times \left( 1.69+1.81 \right)}{\sqrt{3}}=4.041A{}^\circ \\
\end{align}\]
So, the correct answer is “Option B”.
Note: It should be remembered to you that on application of high pressure, The NaCl structure changes to CsCl structure whereas on heating the CsCl structure at 760K, the CsCl structure transforms to NaCl structure.
The structure of NaCl and CsCl is given below:

The black atoms correspond to $C{{l}^{-}}$ ions and white atoms correspond to $N{{a}^{+}}$ atoms.

The grey atoms correspond to $C{{l}^{-}}$ ions and black atom is $C{{s}^{+}}$ ion.
Also, you should remember that CsCl is used in isopycnic centrifugation for separating various types of DNA.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




