
How is cumene converted into phenol? Give a test to distinguish between phenol and ethanol?
Answer
542.1k+ views
Hint :Here we have two questions. We know that Cumene is an organic compound acquired by Friedel-Crafts alkylation of benzene with propylene. That is, Cumene means isopropyl benzene. On oxidation of cumene and on further action we will get phenol. We can distinguish between phenol and ethanol by reaction with neutral $ FeC{l_3} $ .
Complete step by step solution:
Now conversion of cumene to phenol,
On oxidation of cumene (Isopropyl benzene) in the presence of air, cumene hydroperoxide is found. Upon further action of cumene hydroperoxide with dilute acid, phenols are produced.
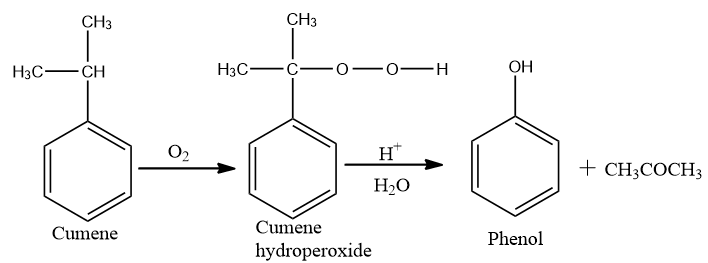
Acetone is also made as one of the by-products of the reaction in large quantities. Therefore, phenols prepared by these techniques need purifications.
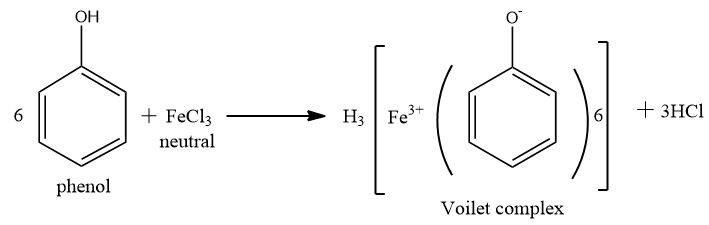
Now distinguish between phenol and ethanol by reaction with neutral $ FeC{l_3} $ .
Phenol reacts with aqueous neutral $ FeC{l_3} $ to form ferric phenoxide complex, which is violet coloured. But ethanol (alcohol) does not respond to $ FeC{l_3} $ .
Note:
We can prepare phenols from haloarenes or from benzene sulphonic acid or from diazonium salts and by many other methods. We can also distinguish phenol and ethanol by haloform test. Ethanol when treated with an iodine, iodoform is obtained which is yellow needle shaped. But phenol does not show haloform test.
$ C{H_3}C{H_2}OH\xrightarrow{{{I_2} + O{H^ - }}}CH{I_3} $ .
Phenol is weakly acidic hence turns blue litmus red. It gives a positive dye test, whereas alcohols (ethanol) do not show dye test.
Complete step by step solution:
Now conversion of cumene to phenol,
On oxidation of cumene (Isopropyl benzene) in the presence of air, cumene hydroperoxide is found. Upon further action of cumene hydroperoxide with dilute acid, phenols are produced.

Acetone is also made as one of the by-products of the reaction in large quantities. Therefore, phenols prepared by these techniques need purifications.
Now distinguish between phenol and ethanol by reaction with neutral $ FeC{l_3} $ .
Phenol reacts with aqueous neutral $ FeC{l_3} $ to form ferric phenoxide complex, which is violet coloured. But ethanol (alcohol) does not respond to $ FeC{l_3} $ .

Note:
We can prepare phenols from haloarenes or from benzene sulphonic acid or from diazonium salts and by many other methods. We can also distinguish phenol and ethanol by haloform test. Ethanol when treated with an iodine, iodoform is obtained which is yellow needle shaped. But phenol does not show haloform test.
$ C{H_3}C{H_2}OH\xrightarrow{{{I_2} + O{H^ - }}}CH{I_3} $ .
Phenol is weakly acidic hence turns blue litmus red. It gives a positive dye test, whereas alcohols (ethanol) do not show dye test.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




