
Cyclohexanone on being heated with $NaOH$ solution forms:
(A).
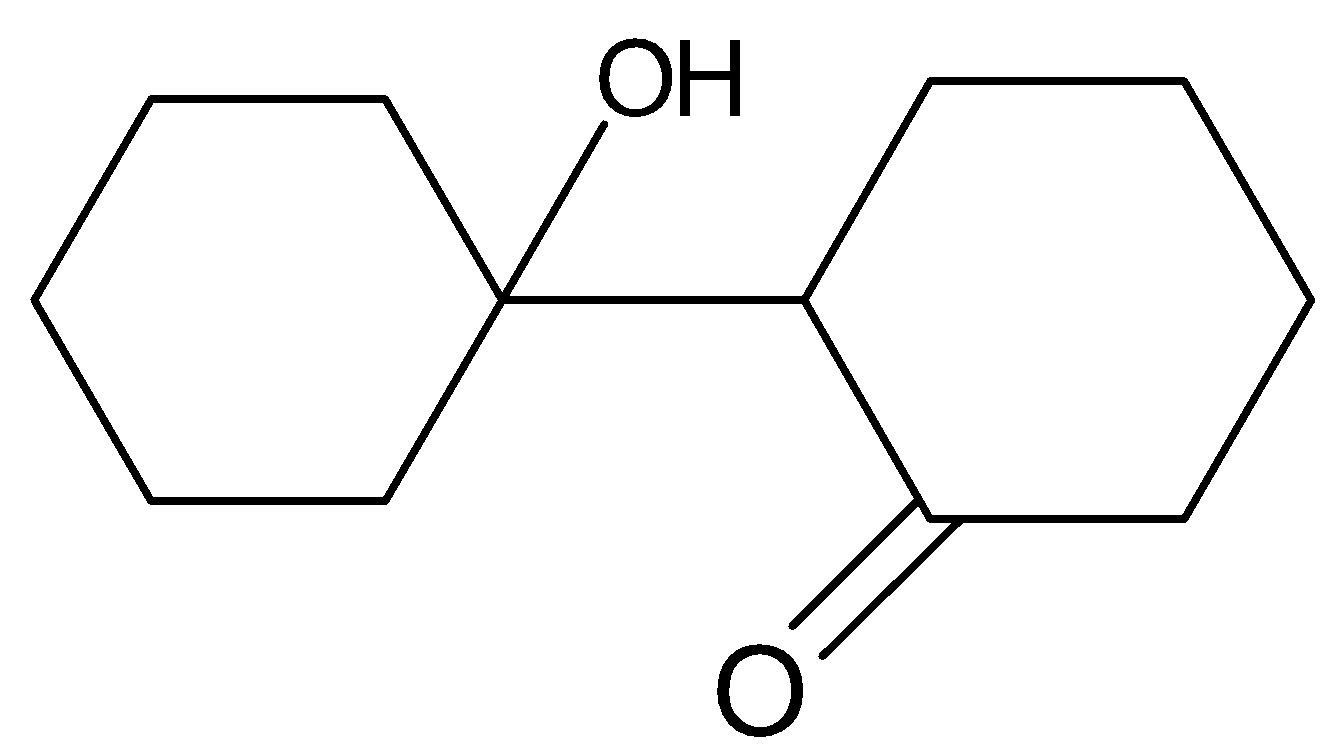
(B).
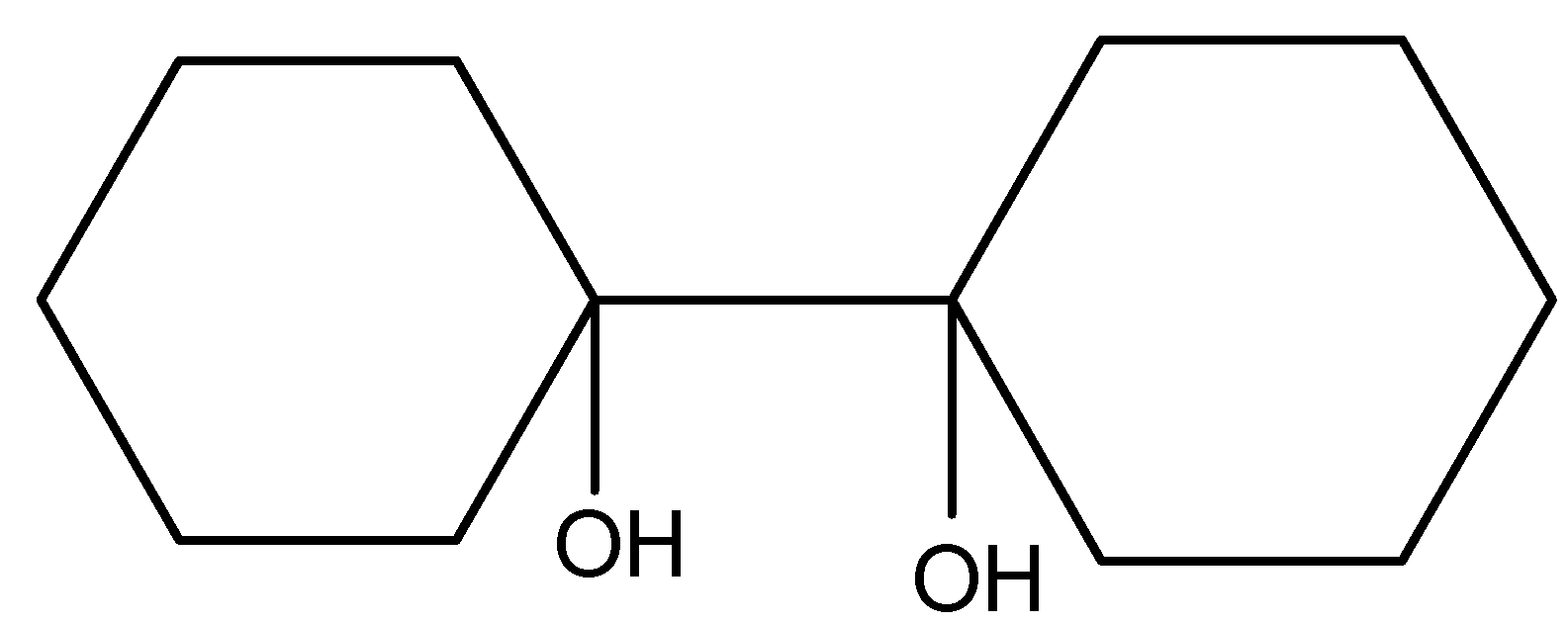
(C).
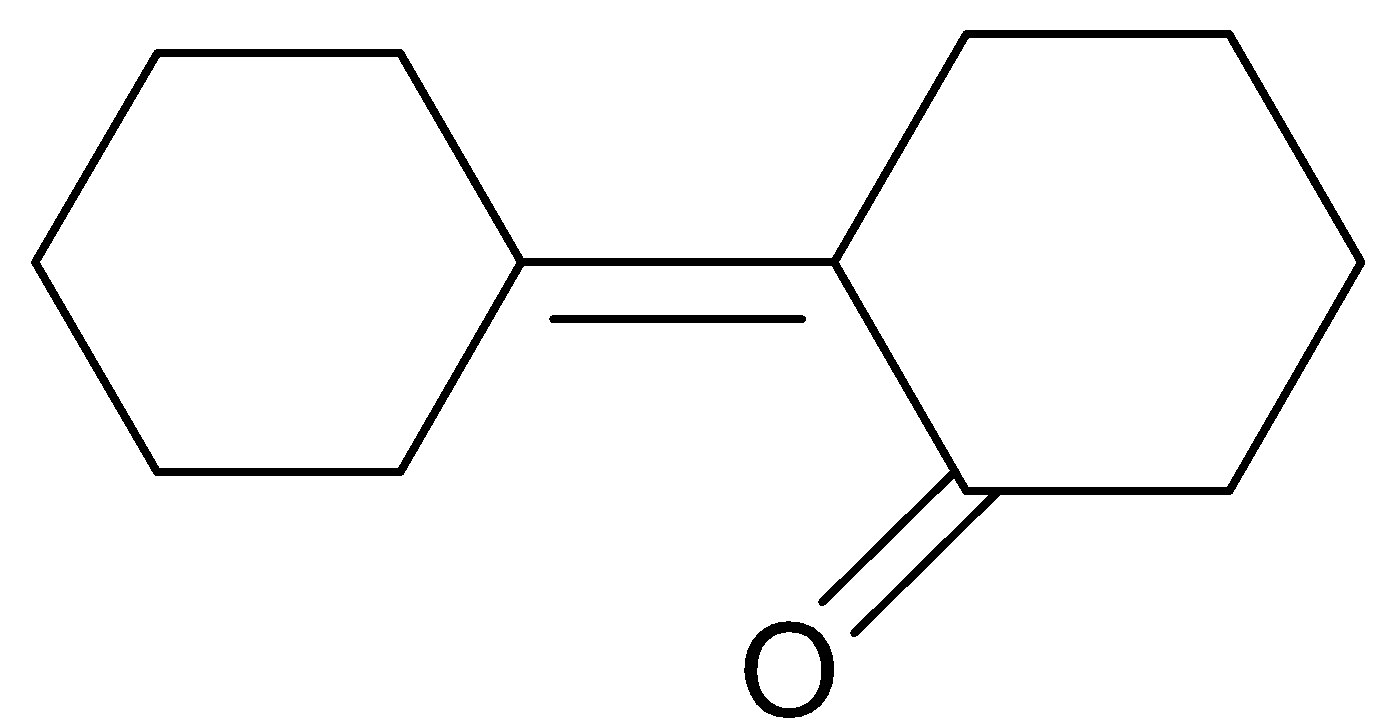
(D).
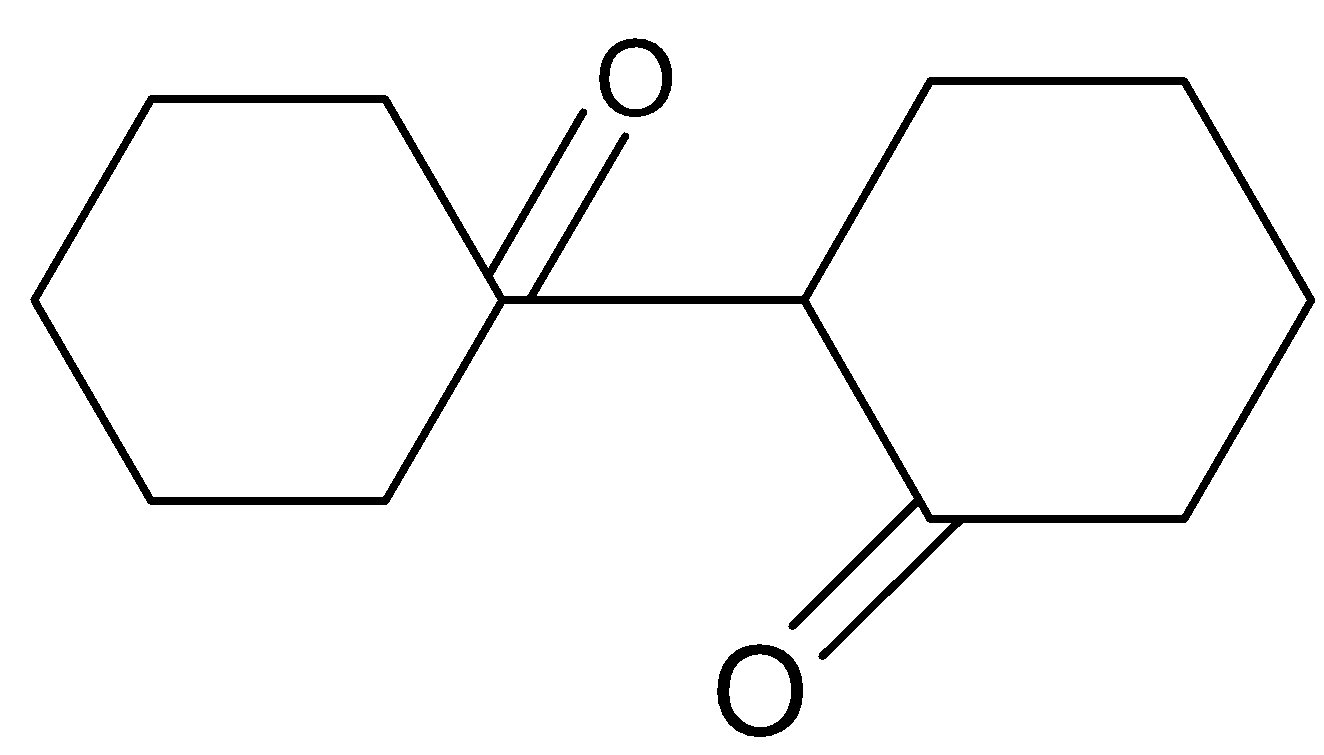




Answer
568.8k+ views
Hint:
Cyclohexanone is an organic cyclic compound. When it is heated with $NaOH$ , it undergoes aldol condensation followed by dehydration to give the required product. Aldol condensation is an organic reaction in which enolate ion reacts with a carbonyl compound to form beta-hydroxy aldehyde followed by dehydration to give enone.
Complete step by step answer:
As we known, cyclohexanone is an organic compound with chemical formula ${({C_2}{H_5})_5}CO$ and structure as:-
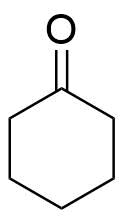
It is a colorless oil with the odour reminiscent of acetone. The vapours of cyclohexanone are heavier than air. The vapours are irritating to the eyes, skin and respiratory tract. Exposure far above the OEL could cause lowering of consciousness. Cyclohexanone samples have yellow colour. It is soluble in water and in organic solvents of ${C_6}{H_{12}}$ cyclohexanone $\left[ {{C_6}{H_{12}}} \right]$ in presence of air.
i.e. ${C_6}{H_{12}} + {O_2} \to {(C{H_2})_5}CO + {H_2}O$
Alternatively it is produced by the partial hydrogenation of phenol.
i.e. ${C_6}{H_5}OH + 2{H_2} \to {(C{H_2})_5}CO.$
The great majority of cyclohexanone is used for the production of Nylon $ 6,6$ and Nylon $6.$ Moreover the cyclohexanone is used in many laboratory reactions. Cyclohexanone reacts vigorously with strong oxidants like nitric acid which results in the formation of a great amount of heat along with hazardous explosion.
When cyclohexanone is heated with $NaOH$ , it proceeds with the mechanism of aldol condensation followed by dehydration. Aldol condensation is a condensation reaction in or enol reacts with a carbonyl compound to form $\beta - $ Hydroxy Aldehyde or $\beta - $ Hydroxy Ketone, followed by dehydration to give a conjugated ion. The reaction of cyclohexanone with $NaOH$ is as follows.
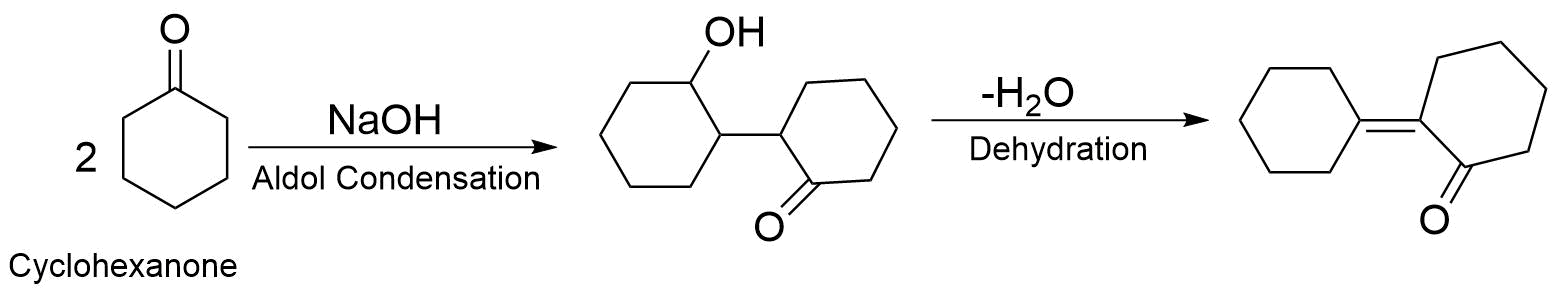
Hence option (C) is a correct answer.
Note: Aldol condensation only takes place if $\alpha - $ Hydrogen, the reaction with $NaOH$ is known as cannizzaro reaction. Moreover in aldol condensation, only diluted $NaOH$ is used. If the condensation reaction occurs between two different carbonyl compounds then it is known as crossed aldol condensation.
Cyclohexanone is an organic cyclic compound. When it is heated with $NaOH$ , it undergoes aldol condensation followed by dehydration to give the required product. Aldol condensation is an organic reaction in which enolate ion reacts with a carbonyl compound to form beta-hydroxy aldehyde followed by dehydration to give enone.
Complete step by step answer:
As we known, cyclohexanone is an organic compound with chemical formula ${({C_2}{H_5})_5}CO$ and structure as:-

It is a colorless oil with the odour reminiscent of acetone. The vapours of cyclohexanone are heavier than air. The vapours are irritating to the eyes, skin and respiratory tract. Exposure far above the OEL could cause lowering of consciousness. Cyclohexanone samples have yellow colour. It is soluble in water and in organic solvents of ${C_6}{H_{12}}$ cyclohexanone $\left[ {{C_6}{H_{12}}} \right]$ in presence of air.
i.e. ${C_6}{H_{12}} + {O_2} \to {(C{H_2})_5}CO + {H_2}O$
Alternatively it is produced by the partial hydrogenation of phenol.
i.e. ${C_6}{H_5}OH + 2{H_2} \to {(C{H_2})_5}CO.$
The great majority of cyclohexanone is used for the production of Nylon $ 6,6$ and Nylon $6.$ Moreover the cyclohexanone is used in many laboratory reactions. Cyclohexanone reacts vigorously with strong oxidants like nitric acid which results in the formation of a great amount of heat along with hazardous explosion.
When cyclohexanone is heated with $NaOH$ , it proceeds with the mechanism of aldol condensation followed by dehydration. Aldol condensation is a condensation reaction in or enol reacts with a carbonyl compound to form $\beta - $ Hydroxy Aldehyde or $\beta - $ Hydroxy Ketone, followed by dehydration to give a conjugated ion. The reaction of cyclohexanone with $NaOH$ is as follows.

Hence option (C) is a correct answer.
Note: Aldol condensation only takes place if $\alpha - $ Hydrogen, the reaction with $NaOH$ is known as cannizzaro reaction. Moreover in aldol condensation, only diluted $NaOH$ is used. If the condensation reaction occurs between two different carbonyl compounds then it is known as crossed aldol condensation.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




