
When cyclohexanone reacts with selenium dioxide, what product will be formed?
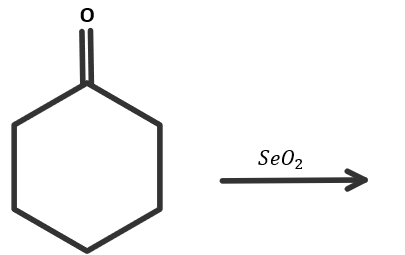
A.Cyclohexane $1,2 - $ dione
B.$1,6 - $ hexanedione
C.Cyclohexane $1,4 - $ dione
D.$2 - $ hydroxy cyclohexan $1 - $ one

Answer
575.7k+ views
Hint: Functional group: In the hydrogen the atoms or groups which are other than carbon and hydrogen, are known as function groups. For example: chloride if chlorine is present in the compound. And if $CO$ is attached then the functional group will be ketone.
Complete step by step solution:
First of all we will talk about the alkanes, alkenes and alkynes.
Alkanes: The compounds which are formed by carbon and hydrogen and have only a single bond between the carbon-carbon atoms, are known as alkanes. For example: The first member of the alkane family is ethane $({H_3}C - C{H_3})$. The general formula of the alkane group is ${C_n}{H_{(2n + 2)}}$.
Alkenes: The compounds which are formed by carbon and hydrogen and have at least one double bond along with a single bond between the carbon-carbon atoms, are known as alkenes. For example: The first member of the alkene family is ethene $({H_2}C = C{H_2})$. The general formula of the alkene group is ${C_n}{H_{2n}}$.
Alkynes: The compounds which are formed by carbon and hydrogen and have at least one triple bond along with a single bond between the carbon-carbon atoms, are known as alkynes. For example: The first member of the alkyne family is ethyne $(HC \equiv CH)$. The general formula of the alkyne group is ${C_n}{H_{(2n - 2)}}$.
Functional group: In hydrogen the atoms or groups which are other than carbon and hydrogen, are known as function groups. For example: chloride if chlorine is present in the compound.
When cyclohexanone reacts with selenium dioxide, then the product is as Cyclohexane $1,2 - $ dione. Because the cyclohexane ring will be the same and one more ketone group will be attached to the parent chain so the IUPAC name of the product will be Cyclohexane $1,2 - $ dione. The reaction is as follows:
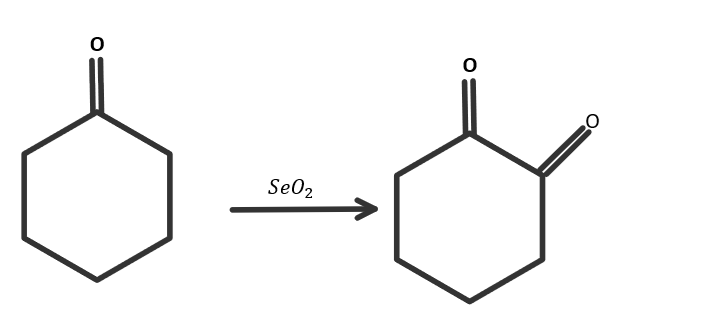
So the correct answer is option C.
Note: Suffix to some functional group are as: for carboxylic acid suffix used is –oic acid, for alcohols suffix used is alkyl alcohol. For example: if an alcohol group is present in methane then the IUPAC name of the compound will be methyl alcohol.
Complete step by step solution:
First of all we will talk about the alkanes, alkenes and alkynes.
Alkanes: The compounds which are formed by carbon and hydrogen and have only a single bond between the carbon-carbon atoms, are known as alkanes. For example: The first member of the alkane family is ethane $({H_3}C - C{H_3})$. The general formula of the alkane group is ${C_n}{H_{(2n + 2)}}$.
Alkenes: The compounds which are formed by carbon and hydrogen and have at least one double bond along with a single bond between the carbon-carbon atoms, are known as alkenes. For example: The first member of the alkene family is ethene $({H_2}C = C{H_2})$. The general formula of the alkene group is ${C_n}{H_{2n}}$.
Alkynes: The compounds which are formed by carbon and hydrogen and have at least one triple bond along with a single bond between the carbon-carbon atoms, are known as alkynes. For example: The first member of the alkyne family is ethyne $(HC \equiv CH)$. The general formula of the alkyne group is ${C_n}{H_{(2n - 2)}}$.
| Number of carbon atom in alkane | Name of the parent chain |
| One | Methane |
| Two | Ethane |
| Three | Propane |
| Four | Butane |
| Five | Pentane |
| Six | Hexane |
| Seven | Heptane |
Functional group: In hydrogen the atoms or groups which are other than carbon and hydrogen, are known as function groups. For example: chloride if chlorine is present in the compound.
When cyclohexanone reacts with selenium dioxide, then the product is as Cyclohexane $1,2 - $ dione. Because the cyclohexane ring will be the same and one more ketone group will be attached to the parent chain so the IUPAC name of the product will be Cyclohexane $1,2 - $ dione. The reaction is as follows:

So the correct answer is option C.
Note: Suffix to some functional group are as: for carboxylic acid suffix used is –oic acid, for alcohols suffix used is alkyl alcohol. For example: if an alcohol group is present in methane then the IUPAC name of the compound will be methyl alcohol.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




