
Deduce the structure of $P{F_5}$ on the basis of $VSEPR$ theory.
Answer
567k+ views
Hint: $VSEPR$ theory is a very important theory to predict the geometry or shape of a number of polyatomic molecules or ions of non-transition elements. This theory was proposed by Sidwick and powell in $1940.$
Complete answer:
$VSEPR$ theory assumes that these electron pairs ( bond pairs as well as lone pairs) occupy localized orbitals which arrange themselves in space in such a way they keep apart from one another as far as possible so that they may experience less electrostatic repulsion between them.
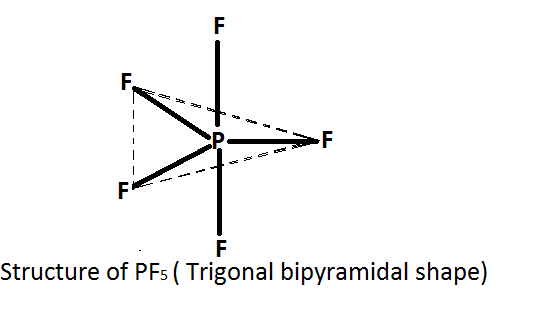
In the given question we have to deduce the structure of $P{F_5}$ . According to this theory if the central atom is surrounded by five electron pairs ( bond pairs as well as lone pairs) , then these electrons are directed towards the corner of trigonal bipyramidal.
We know in $P{F_5}$ the central atom is phosphorus \[\left( P \right)\] and it is surrounded by five fluorine $\left( F \right)$ atoms. Thus it forms five bonds with fluorine atoms and no lone pairs present on it. Therefore according to the $VSEPR$ theory $P{F_5}$ has trigonal bipyramidal structure.
 Note:
Note:
Valence Shell Electron Pair Repulsion Theory $\left( {VSEPR} \right)$ .This is a very useful theory to predict the geometry or shape of a number of polyatomic molecules or ions on a non-transition element. This theory says that shapes of a species depend on the number of and nature of electron pairs surrounding the central atom of a species.
Table summarizes the relationship between the number of electron pairs that are bond pairs and lone pairs and the shape or geometry.
Complete answer:
$VSEPR$ theory assumes that these electron pairs ( bond pairs as well as lone pairs) occupy localized orbitals which arrange themselves in space in such a way they keep apart from one another as far as possible so that they may experience less electrostatic repulsion between them.
In the given question we have to deduce the structure of $P{F_5}$ . According to this theory if the central atom is surrounded by five electron pairs ( bond pairs as well as lone pairs) , then these electrons are directed towards the corner of trigonal bipyramidal.
We know in $P{F_5}$ the central atom is phosphorus \[\left( P \right)\] and it is surrounded by five fluorine $\left( F \right)$ atoms. Thus it forms five bonds with fluorine atoms and no lone pairs present on it. Therefore according to the $VSEPR$ theory $P{F_5}$ has trigonal bipyramidal structure.

Valence Shell Electron Pair Repulsion Theory $\left( {VSEPR} \right)$ .This is a very useful theory to predict the geometry or shape of a number of polyatomic molecules or ions on a non-transition element. This theory says that shapes of a species depend on the number of and nature of electron pairs surrounding the central atom of a species.
Table summarizes the relationship between the number of electron pairs that are bond pairs and lone pairs and the shape or geometry.
| Total number of electron pairs | Shape |
| 2 | Linear |
| 3 | Trigonal planar |
| 4 | Tetrahedral |
| 5 | Trigonal bipyramidal |
| 6 | Octahedral |
| 7 | Pentagonal bipyramidal |
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

What is a transformer Explain the principle construction class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE




